In recent years dentistry has advanced from more than just oral health to overall health. One of the outcomes of this is dental sleep medicine – the management of snoring and obstructive sleep apnoea (OSA), both of which are classed as a sleep-related breathing disorder (SRBD). Approximately one in 30 people in the UK complain about headaches each year and it is estimated that around 1.5 million adults have OSA, although only around 330,000 are currently diagnosed and treated. These numbers are increasing as obesity levels rise in adults and children. In a UK cross-sectional study, 12 per cent of children were found to be habitual snorers and 0.7 per cent were found to have obstructive sleep apnoea 1.
Understanding sleep apnoea and snoring
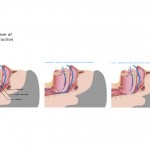
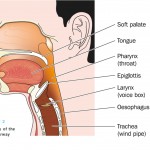
OSA is a sleep-related respiratory condition, leading to intermittent cessations of breathing due to a narrowing or closure of the upper airway during sleep. Symptoms of OSA often include excessive daytime sleepiness, snoring, and witnessed apnoeas or hypopnoeas (collapse of the airway leading to breathing cessations). Although OSA is thought to be a disorder affecting the overweight or obese, it can affect anyone and is estimated to affect 1.5 million adults in the UK, men, women and children.
Craniofacial pain and TMD
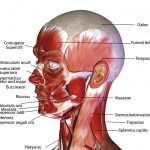
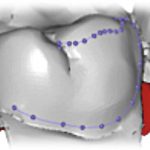
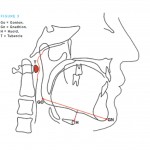
Temporomandibular joint dysfunction (TMD) refers to a group of disorders affecting the temporomandibular joint (TMJ), masticatory muscles and the associated structures (Fig 1). Common symptoms of TMD include pain, limited mouth opening and joint noises (also known as clicking of the jaw).
TMD symptoms affect up to 25 per cent of the population with only 5 per cent seeking medical help for their symptoms – they simply put up with the pain or can’t find a treatment 2. TMD can occur at any age but is more common among women and those between the ages of 20 and 50.
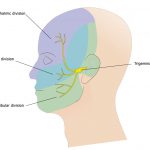
The main sensory nerve system running through the head is the trigeminal nerve system (Fig 2) and accounts for 90 per cent of all the sensory input into the entire nervous system. Because of this we can explain why TMD can sometimes lead to debilitating symptoms for those who suffer from this condition. Many patients will seek treatment for craniofacial pain due to recurring migraines, but 90 per cent of headaches are really caused by disorders in the facial muscles and nerves.
- Figure 1 – Muscles of the face including the masticatory muscles
- Figure 2 – Trigeminal nerve – the three different innervation areas of the head
- Figure 3 – Continuous positive airway pressure (CPAP) machine in use
A connection between sleep apnoea and pain
As research continues to advance we see a clear connection between sleep-disordered breathing, craniofacial pain and TMD, which requires proper evaluation and diagnosis by dental and medical teams. Essentially, it is the dental clinician who will often evaluate, refer and possibly manage these issues which impact such a large percentage of the population.
There is ample evidence to suggest that sleep apnoea and pain are related, but many questions still remain. One main trend emerging pertains to the directionality and mechanisms of the association of sleep apnoea and chronic pain. It appears that sleep disturbance may impair key processes that contribute to the development and maintenance of chronic pain, including joint pain (TMD). In a recent study, sleep disturbance and pain were connected. It determined that pain not only has direct effects on the person’s health, but also an association with sleep disturbances
Many studies have suggested that experimental sleep disruption results in enhanced pain perception and interactions between sleep and pain. It is suggested that experimental sleep disruption results in enhanced pain perception, that poor sleep is correlated with elevated pain severity in chronic pain patients and that in the general population, individual differences in sleep impact on subsequent pain. A study published in the European Journal of Pain stated that sleep fragmentation among healthy adults resulted in subsequent decrements in endogenous pain inhibition 3.
With an evident relationship, we look to understand that clenching or grinding of one’s teeth is a way for the brain to protect itself from suffocation during sleep 4,5,6,7,8. The screening process is important in helping us identify bruxism as either a cause of TMJ/craniofacial pain or a protective mechanism in sleep disordered breathing 9,10,11,12. By identifying this link between the three conditions we can properly manage each disorder.
Dental solutions for proper treatment
Patients who suffer from severe sleep apnoea might opt for surgery for treatment. However, sleep apnoea surgeries have a history of causing the patient excruciating pain. The gold standard for severe OSA patients is use of the continuous positive airway pressure (CPAP) machine (Fig 3), with success ranging in various studies from 90 to 95 per cent. However, the problem with CPAP treatment is patient non-compliance and intolerance. When people return home, there is a good chance they just won’t use their machine 13.
There are challenges posed by sleep apnoea and craniofacial pain which span the research spectrum – from causes to diagnosis through treatment and prevention. It is important for us all to work together to gain a better understanding of sleep apnoea, the TMJ and muscle disease process and craniofacial pain, as well as improving quality of life for people affected by these disorders.
Dentists see their patients more often than family doctors, as it is recommended that patients visit a dentist at least twice a year. Since we are typically seeing our patients more often, it is important to understand sleep apnoea, TMD and craniofacial pain, as well as gaining an understanding of the right questions to ask.
As only one out of 20 UK patients suffering from pain actually seek treatment and that 85 per cent of patients with sleep apnoea either don’t or do not know where to seek help, it is vital that we ask the right questions in order to gain a proper diagnosis. If they are suffering from pain they might not realise the solution can be found at the dental practice:
- Palliative care – medications to better improve a patient’s pain
- Changing a patient’s diet – this would include soft foods or foods that don’t overextend the jaw or cause pressure on the head. For sleep apnoea it would include foods to help in weight control or loss
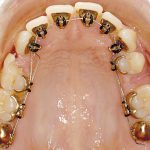
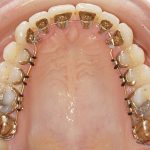
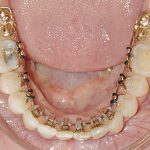
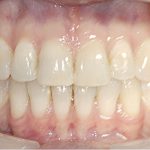
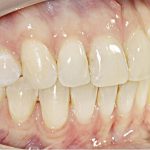
- Oral appliance therapy and orthodontics – offer a way to realign the jaw and teeth to relieve pressure on the face and jaw while preventing the tongue from falling over the airway.
Dentists hold the key to successful management of sleep apnoea and pain among patients who might think a solution is not possible. It is our responsibility to continue to advance our knowledge of various areas of dentistry we might not be exposed to in our undergraduate training – there is more out there than we were taught.
Advanced education
As dentists we must look to better understand sleep apnoea, snoring and craniofacial pain in order to provide our patients with the care they need to live a good quality of healthy and happy lives. Further education through lectures and seminars becomes essential with a wide range of continuing education courses available. Only through education can we continue to offer the help that patients with this debilitating condition need.
Since this is not a subject that is covered in the undergraduate curriculum, postgraduation certification in dental sleep medicine and craniofacial pain allows the whole team to engage with various medical and dental specialities to offer the optimum management options to these patients.
The British Society of Dental Sleep Medicine (BSDSM) has for many years run one-day dental sleep medicine courses which have proved very popular. As well as providing an overview of sleep disordered breathing, advising how to identify and safely assess patients at risk and explaining treatment and management options, the courses now include a module on temporomandibular joint dysfunction and craniofacial pain.
The BSDSM also provides a wealth of information on snoring and OSA, advice on treatment options for the public, patients and healthcare professionals and promotes discussion on dental sleep medicine. For more information, visit www.bsdsm.org.uk
About the author
Dr Mayoor Patel DDS, MS, D.ABDSM, DABOP, DABCP, DABCDSM, DAPPM, RPSGT, FAAOP, FICCMO, FAACP, FAGD is owner of the Craniofacial Pain Center of Georgia in the US, co-owner of MAP Laboratory LLC and Director of Clinical Education at Nierman Practice Management. He is a board member of the American Board of Craniofacial Pain, the American the Academy of Craniofacial Pain, the American Board of Craniofacial Dental Sleep Medicine and a director of the Georgia Association of Sleep Professionals.
Dr Aditi Desai BDS, MSc is president of the British Society of Dental Sleep Medicine (BSDSM). She has accreditation from the European Academy of Dental Sleep Medicine and serves on the Council of the Odontological and Sleep Section at the Royal Society of Medicine (RSM). Dr Desai limits her practice predominantly to the management of sleep disorders. Based in Harley Street and London Bridge Hospital, she works with other eminent physicians and ENT consultants as part of a multidisciplinary team of like-minded professionals with special interest in sleep medicine. She is an invited speaker at the RSM, the Royal College of Surgeons of England, the British Sleep Society, the British Dental Association and many other organisations. She has published several articles in dental journals on dental sleep medicine and lectures on the subject in the UK and internationally.
References
1. Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis Child 1993; 68(3): 360-6.
2. Murphy, M.K., et al., Temporomandibular Joint Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. The International Journal of Oral & Maxillofacial Implants, 2013. 28(6): p. e393.
3. Edwards, R., et al., Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. European Journal of Pain, 2009. 13(10): p. 1043-1047.
4. Ng, D.K., et al., Prevalence of sleep problems in Hong Kong primary school children: A community-based telephone survey. Chest, 2005. 128(3): p. 1315-1323.
5. Phillips, B., et al., Effect of sleep position on sleep apnea and parafunctional activity. Chest, 1986. 90(3): p. 424-429.
6. Bailey, D.R., Sleep disorders. Overview and relationship to orofacial pain. Dental clinics of North America, 1997. 41(2): p. 189-209.
7. Hosoya, H., et al., Relationship between sleep bruxism and sleep respiratory events in patients with obstructive sleep apnea syndrome. Sleep Breath, 2014. 18(4): p. 837-44.
8. Saito, M., et al., Temporal association between sleep apnea-hypopnea and sleep bruxism events. J Sleep Res, 2013.
9. Blanco Aguilera, A., et al., Relationship between self-reported sleep bruxism and pain in patients with temporomandibular disorders. J Oral Rehabil, 2014. 41(8): p. 564-72.
10. Manfredini, D. and F. Lobbezoo, Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 2010. 109(6): p. e26-e50.
11. Unell, L., et al., Changes in reported orofacial symptoms over a 10-year period as reflected in two cohorts of 50-year-old subjects. Acta Odontol Scand, 2006. 64(4): p. 202-8.
12. Ahlberg, K., et al., Perceived orofacial pain and its associations with reported bruxism and insomnia symptoms in media personnel with or without irregular shift work. Acta Odontol Scand, 2005. 63(4): p. 213-7.
13. Richard, W., et al., Acceptance and long-term compliance of nCPAP in obstructive sleep apnea. European Archives of Oto-Rhino-Laryngology, 2007. 264(9): p. 1081-1086.
Make no mistake, we’re no longer mimicking the US culture of litigation – we’re now leading the world. So, whether you’re a dentist, a doctor, or in the business of offering legal representation to either, it’s well worth understanding just who’s driving this financially and emotionally expensive cultural revolution. Is there a particular species of litigious patient, and if so, what do they look like?
The answer is obviously ‘no’, but there are certainly patterns of behaviour and traits of character that have become apparent to me during more than 30 years as a restorative dentist, and as an expert witness with experience of both sides of the judicial fence. Of course, it’s preferable to avoid any patient-practitioner relationship developing into full-blown litigation in the first place. Complaints invariably originate from poor communication, and can be provoked by what is said, is not said, or both.
My own audit of more than 20 years of documentation revealed the following triggers that led to the patients issuing civil proceedings, in descending order of frequency:
1. A sense of abandonment and failure to respond when problems became apparent.
2. Miscommunication with an English-speaking patient due to the clinician’s mother tongue not being English.
3. Failure to identify that the clinician is out of his/her depth and a referral to a colleague clearly indicated.
4. The absence of the usual clinician due to illness or holiday, causing the patient to attend another practitioner who makes a negative comment about their dental state, eg untreated periodontal disease or tooth decay.
5. Failure to adhere to well-established clinical protocols, with specific reference to alternative therapies with no scientific or clinical data to support their use.
6. Early failure of the treatment with unsuccessful efforts to resolve the problems.
7. A report that the clinician was too brusque and/or rude and appeared in a hurry.
8. Patients reporting that the clinician lost his cool/patience and/or shouted at them.
It often transpires that the ‘offending’ practitioner has chivvied a patient into making a choice using demanding or dictatorial language, whether real or perceived. A recent review of complaints carried out by one of the leading dental defence indemnity insurers concluded that more than 70 per cent of complaints were attributed to poor communication, highlighting not only indelicate vocabulary but the manner of delivery and body language, suggesting a lack of ‘feeling’ or compassion.
In contrast – and in a great many of the cases I’ve encountered – clinicians attempt to abandon the patient by not responding to letters of complaint and not returning phone calls. An apology, accompanied by some shared expression of concern and regret, plus an assurance that the problem will be rectified, is often all that’s needed to prevent the matter escalating and the patient taking their grievance to a third party.
Unfortunately, with the best will in the world, clear communication sometimes isn’t enough and the motive of revenge or financial gain can mean a practitioner’s reasonable defence falls on deaf ears. When financial compensation is an unlikely outcome, I’ve observed that writing to governing authorities can become the means by which some patients aim to ‘get back’ at the clinician, ‘teaching them a lesson’ and ‘protecting others from harm’.
I’m confident that patients particularly prone to this course of action have an identifiable character profile. Patientes Litigiosum is almost inevitably female and over 50 years of age. Before I’m accused of sexism, my own audit revealed that 90 per cent of our own claimants – those bringing formal suits against my own clinic – were female. This can be explained by the higher percentage of female patients with long-standing prosthodontic issues referred to the clinic. But a review of all our medico-legal referrals to me as an expert witness and involving litigation suits against general dental practitioners over the last three years revealed a 60 per cent female bias.
Figure 1
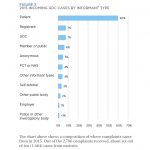
Issues considered by the GDC’s PCC/PPC in 2015
| ISSUE | NUMBER OF OCCURRENCES** | % OF TOTAL OCCURRENCES |
|---|---|---|
| Poor treatment | 179 | 23% |
| Poor record keeping | 111 | 14% |
| Failure to take appropriate radiographs or to interpret | 80 | 10% |
| Fraud/dishonesty | 53 | 7% |
| Failure to obtain consent/ explain treatment | 42 | 5% |
| Failure to cooperate with the GDC or failure to disclose convictions/cautions | 38 | 5% |
| Personal behaviour | 33 | 4% |
| Prescribing issues | 31 | 4% |
| Working outside scope of practice | 25 | 3% |
| No professional indemnity insurance of failing to produce evidence | 24 | 3% |
| Undiagnosed/untreated caries | 21 | 3% |
| Cross-infection control | 16 | 2% |
| Failings in recording medical and/or dental history | 15 | 2% |
| Conviction or caution – other | 14 | 2% |
| Misleading advertising | 11 | 2% |
| Conviction or caution – assault | 10 | 2% |
| Indecent assault or inappropriate sexual behaviour | 10 | 1% |
| Misled about treatment available on the NHS | 9 | 1% |
| Conviction or caution – alcohol or drugs | 9 | 1% |
| Conviction or caution – theft/robbery | 8 | 1% |
| Failure to refer | 7 | <1% |
| Period of unregistered practice | 5 | <1% |
| Clinically incorrect extractions | 4 | <1% |
| Failure to anaesthetise | 4 | <1% |
| Failure to spot or monitor lesions | 3 | <1% |
| Failure to inform patient of adverse incident | 3 | <1% |
| Employing dentist or nurse not registered with GDC | 3 | <1% |
| Inaccurate statements to CQC | 3 | <1% |
| Tooth whitening | 2 | <1% |
| Making racially offensive comments | 1 | <1% |
| Not supervising Vocational Dental Practitioners adequately | 1 | <1% |
| Total | 775 | |
** Cases often involve more than one issue. These figures provide a profile reflecting the main issues involved, and not every single charge
Our litigant is invariably living alone or estranged from partner or family. If married, their relationships have become unloving and burnt out. It is highly likely they are possessed of a long mental health history of chronic anxiety and depressant illness previously treated with medication and/or cognitive behavioural therapy. Expect a high display of feelings when questioned during a consultation appointment. One will also observe multiple functional disorders, including gynaecological complaints, chronic fatigue syndrome, irritable bowel syndrome, and other ailments that long-suffering GPs have failed to ‘put their finger on’. Multiple visits to the GP for exhaustion and irregular sleep patterns are common. The problem for the busy clinician who, understandably, tends to focus on his/ her anatomical area of interest, can easily miss these traits. After all, the dentist is concentrating on the teeth, whereas the orthopaedic surgeon is in ‘bone mode’.
The traditional Western medical approach is collectively disease-focused, whereas the old Greek physician’s philosophy of focusing on “don’t tell me about the disease in the man, but about the man with the disease” could not be closer to the truth. An interview technique that subtly explores the personal, social and professional history is essential in gathering information necessary to the spotting of this high-risk group. Once identified, it then becomes a matter of explaining the interaction of stress and depression upon the immunological competences of a patient, and their ability to cope and heal following stressful surgical assaults. It allows you to share with the patient the responsibility of healing and get them ‘on board’. I have learnt that if a patient can readily connect the dots between their mental and physical health then all is well. However, if the patient vigorously denies any connection between the two, despite it being already abundantly clear, then I consider they’re assuming no responsibility and now refer the patient elsewhere. However, I always pass on this vital piece of information to the referred clinician. Fair’s fair.
- Figure 2
- Figure 3
The logic behind this approach is that I’ve now readily accepted that I cannot possibly connect with all patients all of the time. Where I might fail, another clinician may succeed. By way of an example, a Welsh patient required treatment that she was finding intolerant, and returned repeatedly with bizarre functional symptomology including atypical facial pains and additional locomotor skill loss. A referral to a Welsh consultant did the trick. He carried out a series of placebo adjustments and she reported an extraordinary resolution.
In my opinion, an Englishman was prejudiced from the start, despite the quality of care provided. Incidentally, she had a long history of depressive illness. A useful tool, sadly out of print since 1995 but which I continue to use, is the Cornell Medical Index Questionnaire. This will allow a clinician with no formal training in psychology or psychiatric medicine to identify these patients who often present with multisystem functional disorders.
Having identified the patient traits and context most likely to result in litigation, it seems only fair to consider whether there might be a similar species within the genus Medicus. Is it possible to be more or less prone to action as a practitioner? Although this is wholly anecdotal, I believe that clinicians with OCD characteristics and a liberal sprinkling of Asperger’s make excellent and highly-focused surgeons. But they’re prone to a greater number of complaints when compared with ‘touchy-feely’ clinicians.
Personally, I’d much rather have the indifferent, socially inept OCD character operating on my person than the ‘schmoozer’ who’s more readily distracted by peripheral events. Sadly, as new management practices in the medical arena now demand a more emotional ‘chairside’ manner from our doctors, dentists and surgeons, the public is unaware of what they’re losing. And here’s the rub – for the large part, the rise of the litigious patient helps no-one. The medical profession can maintain the highest possible standards of patient care, but it’s society as a whole that holds blame and scrutiny in balance.
About the author
Toby Talbot is clinical director at the Talbot Clinic. Over the last 17 years, he has established a professional fast-track service for the legal community, helping courts, counsel and judges make accurate and well-informed decisions.
Local anaesthetics interrupt neural conduction by inhibiting the influx of sodium ions through channels within neuronal membranes. When the neuron is stimulated, the channel is activated and sodium ions can diffuse into the cell, triggering depolarisation. Following this sudden change in membrane voltage, the sodium channel assumes an inactivated state and further influx is denied while active transport mechanisms return sodium ions to the exterior.
After this repolarisation, the channel assumes its normal resting state. Local anaesthetics have the greatest affinity for receptors in the sodium channels during their activated and inactivated states rather than when they are in their resting states 1, 2.
Pharmacology
Local anaesthetics consist of three components that contribute necessary clinical properties:
- Lipophilic aromatic ring – improves lipid solubility of the compound
- Intermediate ester/amide linkage
- Tertiary amine.
Articaine consists of an amide group and an ester link. It has a thiophene ring instead of a benzene ring, as seen in the chemical structure of lignocaine. The thiophene ring improves its lipid solubility. Therefore in some studies articaine shows better potential for penetrating through the neuronal sheath and membrane when compared with other local anaesthetics 3.
The dissociation constant of an anaesthetic affects its onset of action. The lower the pKa values, the greater the proportion of uncharged base molecules can diffuse through the nerve sheath. Articaine has a pKa of 7.8, whereas lignocaine has a pKa of 7.9. This proves important when a local anaesthetic is administered to anaesthetise inflamed tissues, where the ph of the tissues is reduced 4. Articaine has a half-life of 20 minutes, whereas lignocaine has a half-life of 90 minutes. Therefore, articaine presents less risk for systemic toxicity during lengthy dental treatments when additional doses of anaesthetic are administered 5.
Comparison between articaine and lignocaine
Some studies argue that there is no significant difference in pain relief provided by 2 per cent lignocaine and 4 per cent articaine where both formulations contain adrenaline 6. However, a recent systematic review demonstrated a different conclusion 7. This review showed that when considering successful infiltration anaesthesia, 4 per cent articaine solution containing adrenaline was almost four times greater than a similar volume of 2 per cent lignocaine also containing adrenaline. Other studies have stated that 4 per cent articaine offers superior levels of anaesthesia in the anterior maxillary region when compared to 2 per cent lignocaine, however this level of superiority appears less significant in the maxillary molar region 8.
There is evidence to support that articaine is more effective in the maxillary posterior region when compared with lignocaine when tissues are inflamed 9. However, there is insufficient evidence to suggest a similar level of superiority for mandibular teeth, where the solution has been administered with the inferior alveolar nerve block technique 10.
The additive administration of lignocaine using the IANB technique and buccal infiltration with articaine could potentially increase the level of pulpal anaesthesia achieved in the mandibular molar and premolar area 11. The inclusion of adrenaline in 4 per cent articaine is considered critical in achieving its profound anaesthesia 12.
Brandt et al demonstrated that articaine was superior when administered using the inferior alveolar nerve block technique (IANB) 7. However, it must be stressed that the potency of the agent administered via the inferior alveolar block was considerably lower than the potency administered by the infiltration technique. It was shown that neither articaine or lignocaine demonstrated superiority over the other when administered to symptomatic teeth. It is important to recognise the limitations in this study of comparing a 4 per cent solution of articaine with a 2 per cent solution of lignocaine 7. Other studies also reported no difference between articaine and lignocaine when using the IANB technique while treating symptomatic teeth 11,13.
Interestingly, it has been demonstrated that 4 per cent articaine with 1:100,000 adrenaline administered using the buccal infiltration technique had a significantly faster onset of pulpal anaesthesia when compared with the inferior alveolar nerve block. Therefore, dentists can consider the use of articaine administered by a buccal infiltration as an alternative to the inferior alveolar nerve block when anaesthetising the mandibular first molar 14. Another study also concluded that articaine delivered by buccal infiltration alone was more effective than lignocaine administered by the inferior alveolar technique when anaesthetising mandibular first molar teeth 15.
Paraesthesia
In 2010, Garisto et al reported 248 cases of paraesthesia after dental treatment 16. Most cases involved mandibular nerve blocks and, in 89 per cent of cases, the lingual nerve was damaged. Paraesthesia was shown to be 7.3 times more likely with 4 per cent articaine when compared with lignocaine. Similar findings were reported by Hillerup et al, who demonstrated greater neural toxicity of 4 per cent compared to 2 per cent articaine. Therefore, it might be advisable to limit the use of 4 per cent articaine to infiltrations and avoid for nerve blocks 17.
Articaine has also been shown to be superior for infiltrations in the mandible and does not cause neural toxicity unless injected near the mental nerve 18.
Paraesthesia has been associated with the use of local anaesthetics, especially when administered using the inferior alveolar nerve block technique 19. Observational research performed in Denmark reported a 20-fold greater risk of nerve injury when articaine was used compared with other local anaesthetics and administered via the IANB technique 17. Given that articaine is less neurotoxic than other anaesthetics, the findings of this research were unexpected 20. It is important to consider that the aetiology of paraesthesia may be the result of a needle injury to the lingual and inferior alveolar nerve. Factors including intra-neural haematoma, extra-neural haematoma, oedema and chemical neurotoxicity of articaine may also play a role 21.
Dentists must also consider the ‘Weber effect’ 22. This occurs when a new product is launched onto the market and is scrutinised more closely. Immediately after 4 per cent articaine containing 1:100,000 adrenaline was introduced, there was a significantly increased incidence of paraesthesia. But, two years later, a reduction was recorded despite an increased number of cartridges being sold 21.
The literature reports that the lingual nerve is more frequently damaged than the inferior-alveolar nerve. Approximately 70 per cent of permanent nerve damage is sustained by the lingual nerve, whereas a 30 per cent occurrence was recorded affecting the inferior alveolar nerve 23. Current data indicates that 85-94 per cent of non-surgical paraesthesia caused by local anaesthetics recovers within two months. After a two month period, two thirds of those patients whose paraesthesia has not resolved will never completely recover 23.
Articaine is also used in areas of medicine such as plastic and reconstructive surgery, ophthalmology and orthopaedic surgery. It is interesting that there are no reports of paraesthesia from articaine following its use in medicine. Is it possible that articaine only affects nerves supplying the oral cavity and specifically the lingual nerve? It is thought that paraesthesia affects the lingual nerve twice as much as the inferior alveolar nerve due to the fascicular pattern of the injection site. Also, when a patient opens their mouth for treatment the lingual nerve is stretched and more anteriorly placed; this decreases its level of flexibility, which is needed to deflect the needle. During administration, the barbed needle can damage the inferior alveolar or lingual nerve during withdrawal 24.
Interestingly, in 2006 – when Hillerup raised concerns that articaine was responsible for neurosensory disturbances – it was found that 80 per cent of all these reports came from Denmark. It is worth noting that, at the time, the Danish population was approximately 5.6 million compared with 501 million in the wider EU community. This research led to the Pharmacovigilance Working Party of the European Union conducting an investigation involving 57 countries and more than 100 million patients treated with articaine. The conclusion was emphatic, stating that all local anaesthetics may cause nerve injury. They estimated that the incidence of sensory impairment following administration of articaine was one in every 4.6 million treated patients. Therefore, no medical evidence existed to prohibit the use of articaine and the safety profile of the drug remained unchanged.
It is worth considering that, before articaine was introduced to the USA, the incidence of permanent nerve damage from inferior alveolar nerve blocks was 1:26,762. In 2007, Pogrel also concluded that nerve blocks can cause permanent damage regardless of which anaesthetic agent is used. Both articaine and lignocaine have been associated with this phenomenon in proportion to their use.
Negative side-effects
Articaine can result in restlessness, anxiety, light-headedness, convulsions, dizziness, tremors, drowsiness and depression 13. Ocular complications have been reported due to interference with sensory and motor pathways 25. Other adverse effects include headaches, facial oedema and gingivitis 13. Skin rashes with itching after administration of articaine have also been cited in the literature 26. Skin necrosis on the chin has also been reported after administration of 4 per cent articaine using the IANB technique 27.
With regards to the cardiovascular system, 4 per cent articaine can decrease cardiac conduction and excitability. Complications such as reduced myocardial contractility, peripheral vasodilation, ventricular arrhythmia, cardiac arrest and, rarely, death have been reported in the literature 28. It is important to exercise caution in patients with severe hepatic impairment. However, the rapid breakdown of articaine into inactive metabolites results in low systemic toxicity 29.
Conclusions on articaine
Since 1973, there have been more than 200 papers published on articaine. Virtually all of these studies have concluded that articaine is as effective and safe as other comparable local anaesthetic agents such as lignocaine, mepivacine or prilocaine. It was shown that articaine is the least likely anaesthetic to induce an overdose caused by administration of too many cartridges. No significant difference in pain relief has been observed between adrenaline containing formulations of 4 per cent articaine and 2 per cent lignocaine.
The time of onset and duration of anaesthesia for 4 per cent articaine is comparable to other commercially available local anaesthetics. Furthermore, the majority of studies have indicated that the incidence of complications including paraesthesia are equal for lignocaine and articaine. The FDA has approved articaine 4 per cent with adrenaline 1:100,000 to age four years in paediatric patients.
The popularity of articaine cannot be disputed within the dental profession. In the USA in 2009, 41 per cent of all dental local anaesthetic used was articaine. In 2012, the market share for articaine in Germany was 97 per cent and in the same year, it was shown that 70 per cent of dentists use articaine in Australia.
Adrenaline-containing anaesthetics
Adrenaline causes constriction of blood vessels by activating alpha-1 adrenergic receptors. It aids hemostasis in the operative field and delays absorption of the anaesthetic. This delayed absorption decreases the risk of systemic toxicity and lengthens its duration of action. Adrenaline can cause considerable cardiac stimulation due to its affect as a beta-1 adrenergic agonist 30.
Cardiovascular influences
Adrenaline is an agonist on alpha, beta-1 and beta-2 receptors. It is a vasoconstrictor as the tiny vessels in the submucosal tissues contain only alpha receptors 31. There is much debate regarding the influence of adrenaline on patients with cardiovascular disease. Dionne et al studied the influence of three cartridges of the American formulation Lidocaine with adrenaline 1:100,000. Submucosal injection of this dosage increased cardiac output, heart rate and stroke volume. Systemic arterial resistance was reduced and mean arterial pressure remained unchanged 32.
Likewise, Hersh et al observed similar results following the administration of articaine containing 1:100,000 and 1:200,000 adrenaline. Although the influence of adrenaline reported by Hersh et al was minor, it is noteworthy that all 14 participants were healthy and taking no medication, yet two of these patients experienced palpitations 33.
A dose of approximately two cartridges of lignocaine containing adrenaline 1:80,000, is the most conservative and frequently cited dose limitation for patients with significant cardiovascular disease. Ultimately, the decision requires the dentist to practise sound clinical judgement and to discuss any concerns with that patient’s doctor if necessary. Peak influences of adrenaline occur within five to 10 minutes following injection and they decline rapidly 33.
Another practical suggestion is to determine the dosage based on patient assessment. If the medical status of a patient is questionable, a sensible protocol is to record baseline heart rate and blood pressure preoperatively and again following administration of two cartridges of lignocaine containing 1:80,000 adrenaline. If the patient remains stable, additional doses may be administered, followed by a reassessment of vital signs 30.
Hypertension
After administering one to two cartridges of adrenaline-containing local anaesthetic with careful aspiration and slow injection and the patient exhibits no signs or symptoms of cardiac alteration, additional adrenaline containing local anaesthetic may be used. A safe option preferred by some dentists is to firstly use a minimal amount of adrenaline-containing local anaesthetic and then supplement as necessary with an adrenaline-free anaesthetic 34.
The risk of the anaesthesia wearing off too soon, resulting in the patient producing elevated levels of endogenous adrenaline because of pain, would be much more detrimental than the small amount of adrenaline in the dental anaesthetic 35.
Drug interactions
Beta-adrenergic blocking drugs increase the toxicity of adrenaline-containing local anaesthetics. It inhibits enzymes in the liver and decreases hepatic blood flow. Therefore, it is advisable not to give large doses of local anaesthetic to patients on beta blockers. There have been multiple reports of stroke and cardiac arrest within the literature 36. Slow administration and aspiration can also help prevent undesirable reactions 37.
Judicious use of adrenaline is recommended for patients medicated with nonselective beta blockers. Unlike selective agents that only block beta-1 receptors on the heart, nonselective agents also block vascular beta-2 receptors. In this case, the alpha agonist action of adrenaline becomes more pronounced and both diastolic and mean arterial pressures can become dangerously increased. This is often accompanied by a sudden decrease in heart rate. Significant consequences of this interaction are well documented 38.
The interaction with beta blockers follows a time course similar to that observed for normal cardiovascular responses to adrenaline. It commences after absorption from the injection site, peaks within five minutes and declines over the following 10-15 minutes. Adrenaline is not contraindicated in patients taking nonselective beta blockers, but doses must be kept minimal and monitoring of blood pressure advisable 39.
Verapamil, which is a popular calcium channel blocker, increases the toxicity of 2 per cent lignocaine. As for patients taking beta-adrenergic blocking drugs, two cartridges should be the limit 40. With regards to bupivacaine, calcium channel blockers enhance the cardiotoxicity of this longer acting anaesthetic 41.
Antihypertensives are the main cardiovascular drugs that interact with anaesthetics containing adrenaline. Theoretically, beta-blockers, diuretics and calcium-channel blockers may all result in adverse reactions when used with adrenaline-containing local anaesthetics 42.
Adrenaline causes alpha and beta-adrenergic agonism. Alpha-adrenoreceptor stimulation results in vasoconstriction of peripheral blood vessels, whereas beta-adrenoreceptor stimulation decreases vascular resistance due to vasodilation of vessels in the liver and muscles, therefore reducing diastolic blood pressure. If beta-effects are blocked, the alpha-adrenergic stimulation leads to an unopposed increase in systolic blood pressure triggering a cerebrovascular accident.
Therefore, if more than one to two cartridges are needed in such patients, adrenaline-free solutions should be administered. An advantage, however, of beta-adrenoreceptor blockers in dental patients is that the heart is protected from the elevation in rate produced by beta-adrenergic stimulation from exogenous adrenaline 43.
Diuretics can affect the metabolic actions of adrenaline. Increased levels of adrenaline reduces the plasma concentration of potassium 44. These reductions have been documented in patients receiving dental local anaesthetics containing adrenaline 45.
In patients undertaking oral surgery procedures who are taking non-potassium-sparing diuretics, there have been incidences of adrenaline-induced hypokalaemia 44. It should remembered that calcium channel blocking drugs may also increase adrenaline-induced hypokalaemia 46.
| AGENT | CONC W/V | CONC MG/ML | MAX DOSE MG/KG | DOSE ML/KG | MG MAX DOSE | CARTRIGES |
|---|---|---|---|---|---|---|
| BUPIVACAINE 0.5% | 0.5 | 5 | 1.3 | 0.26 | 90 | 8 |
| LIDOCAINE 2% | 2 | 20 | 4.4 | 0.22 | 300 | 6 |
| MEPIVACAINE 2% | 2 | 2 | 4.4 | 0.33 | 300 | 6 |
| ARTICAINE 4% | 4 | 40 | 7 | 0.18 | 500 | 5 |
| PRILOCAINE 3% | 3 | 30 | 5 | 4.4 | 400 | 6 |
| MEPIVACAINE 3% | 3 | 30 | 4.4 | 0.15 | 300 | 4 |
| PRILOCAINE 4% | 4 | 40 | 5 | 0.13 | 400 | 4 |
| AGENT | CONCENTRATION | TRADE NAME |
|---|---|---|
| BUPIVACAINE | 0.5% | MERCAINE |
| MEPIVACAINE | 2% | LIGNOSPAN |
| LIDOCAINE | 2% | SCANDONEST |
| PRILOCAINE | 3% | SEPTANEST |
| ARTICAINE | 4% | CITANEST |
| MEPIVACAINE | 3% | SCONDONEST |
| PRILOCAINE | 4% | CITANEST |
Angina pectoris and post-myocardial infarction
The use of adrenaline containing local anaesthetics is advisable as part of a stress reduction protocol. The dosage of the adrenaline should be limited to that contained in two cartridges of lignocaine 2 per cent 1:80,000 adrenaline. For patients with unstable angina, a recent myocardial infarction less than six months previously or a recent coronary artery bypass graft surgery within three months warrant all elective dental treatment to be deferred 47. If emergency treatment is imperative, stress-reduction protocols with anti-anxiety agents are advisable with a limitation of two cartridges of adrenaline containing anaesthetic 48.
As part of a stress reduction protocol, the Wand allows the dentist to administer local anaesthetic with a non-threatening handpiece. The anaesthetic syringe is often the principle cause of stress for patients as it is considered by many as the most uncomfortable part of dental treatment. The Wand helps deliver a computer-regulated flow of anaesthetic that enables pain-free dental anaesthesia for the different types of injections. This can help to make the patient less anxious.
Cardiac dysrhythmia
Elective dentistry should be postponed in patients with severe or refractory dysrhythmias until they are stabilised.
It is safe to limit the local anaesthetic dose to two cartridges of lignocaine 2 per cent containing 1:80,000 adrenaline 49. The use of periodontal ligament or intraosseous injections using an adrenaline-containing local anaesthetic is contraindicated 50.
Congestive heart failure
Patients taking digitalis glycosides, such as digoxin, should be carefully monitored if adrenaline-containing anaesthetics are administered as an interaction between these two drugs can trigger dysrhythmias. Patients taking long-acting nitrate medications or taking a vasodilator medication may show decreased effectiveness of the adrenaline and therefore may experience a shorter duration of dental local anaesthesia 48.
Cerebrovascular accident
Following a stroke, it is recommended that dental treatment be deferred due to the significantly elevated risk of recurrence. Following a six-month interval, dental procedures can be rescheduled with the use of adrenaline-containing local anaesthetics. If the stroke patient has associated cardiovascular problems, the dosage of local anaesthetic with vasoconstrictor should be kept to a minimum 48.
Asthma
Stress can precipitate an asthma attack, making stress-reduction protocols essential. Conservative use of local anaesthetics containing adrenaline is advised. The Food and Drug Administration warn that drugs containing sulfites can cause allergic reactions in susceptible individuals 51.
Some studies suggest that sodium metabisulfite, which is an antioxidant agent used in dental local anaesthetic, may induce asthma attacks 52. Data is limited on the incidence of this reaction and even in sulfite-sensitive patients, it appears to be an extremely small risk. Indications are that more than 96 per cent of asthmatics are not sensitive to sulfites and those who are sensitive are usually severe, steroid-dependent asthmatics 53.
Perusse and colleagues concluded that local anaesthetic with adrenaline can be safely used in patients with nonsteroid-dependent asthma. However, until we learn more about the sulfite sensitivity threshold, conservative use of local anaesthetic with adrenaline in corticosteroid-dependent asthma patients is advisable. This is due to their higher risk of sulfite allergy and the possibility that an unintentional intravascular injection might occur, causing a severe asthmatic reaction in a sensitive patient 54. However, in recent times, the results of these older studies have been regarded as questionable by many in the profession.
Hepatic disease
In patients with chronic active hepatitis or with carrier status of the hepatitis antigen, local anaesthetic doses must be kept to a minimum. In patients with more advanced cirrhotic disease, metabolism of local anaesthetics may be significantly slowed, resulting in increased plasma levels and complications from toxicity reactions. Local anaesthetic dosage may need to be decreased and the time lapse between injections extended 55.
Diabetes
Some patients experience dramatic swings between hyperglycemia and hypoglycemia and, therefore, the use of adrenaline-containing anaesthetics should be reduced due to the risk of adrenaline-enhanced hypoglycemia 48.
Cocaine
The major concern in patients abusing cocaine is the significant danger of myocardial ischemia, cardiac dysrhythmias and hypertension. Some researchers recommend deferral of dental treatment for 24 to 72 hours 56.
Tricyclic antidepressants
One to two cartridges of adrenaline-containing local anaesthetic can be safely administered to patients taking these drugs. However, careful observation at all times for signs
of hypertension is necessary due to enhanced sympathomimetic effects 57.
HIV
Protease-inhibitor drugs have been shown to increase the plasma levels of lignocaine potentially increasing cardiotoxicity 58.
Parkinson’s disease
Athough there is no data regarding the influence of the anti-Parkinson drug entacapone, caution is advised while using adrenaline-containing anaesthetics. Three cartridges of 2 per cent lignocaine with 1:80,000 adrenaline is the recommended upper limit in adults 59.
Local anaesthetic reversal
A local anaesthetic reversal agent has been introduced that effectively reverses the influence of adrenaline on submucosal vessels. Phentolamine (Ora Verse) is an alpha receptor blocker formulated in dental cartridges 60.
In the future, this may prove useful for some medically compromised patients such as diabetics or elderly patients for whom adequate nutrition may be hindered by prolonged numbness. However, currently this reversal agent is not available in the UK or Ireland.
About the author
Dr Laura Fee graduated with an honours degree in dentistry from Trinity College, Dublin. During her studies, she was awarded the Costello medal for undergraduate research on cross-infection control procedures. She is a member of the Faculty of Dentistry at the Royal College of Surgeons and, in 2013, she completed the Certificate in Implant Dentistry with the Northumberland Institute of Oral Medicine and has since been awarded the Diploma in Implant Dentistry with the Royal College of Surgeons, Edinburgh. Laura is currently completing the Certificate in Minor Oral Surgery with the Royal College of Surgeons, England. She has also been involved with undergraduate teaching in the School of Dentistry, Belfast where she has an honorary oral surgery contract.
References
-
Berde CB, Strichartz GR. Local anesthetics. In: Miller RD, Eriksson LI, Fleisher LA et al. Miller’s Anesthesia. 7th ed. Philadelphia, Pa: Elsevier, Chuchill Livingstone, 2009.
-
Katzung BG, White PF. Local anesthetics. In:Katzung BG, Masters SB, Trevor AJ, editors. Basic and Clinical Phamacology. 11th ed. New York, NY: Mc Graw-Hill Companies Inc; 2009
-
Malamed SF, Gagon S, Leblanc D. The efficacy of articaine: a new amide local anesthetic. J Am Dent Assoc 2000; 131:635-642
-
Kakroudu SHA, Mehta S, Millar BJ. Articaine Hydrochloride: Is it the solution? Dent Update 2015; 42:88-93
-
Becker DE, Reed KL. Essentials of local anesthetic pharmacology. Anesth Prog 2006; 53(3):98-108
-
Malamed SF. The periodontal ligament injection: an alternative to inferior alveolar nerve block. Oral Surg 1982; 53:117
-
Brandt R, Anderson P, Mc Donald N, Sohn W, Peters M. The pulpal anaesthetic efficacy of articaine versus lidocaine in dentistry. J Am Dent Assoc 2011; 142(5):493-504
-
Yapp K, Hopcraft M, Parashos P. Articaine: a review of the literature. Br Dent J 2011; 210:323-329
-
Srinivasan N, Kavitha M, Longanathan C, Padmini G. Comparison of the efficacy of 4% articaine and 2% lidocaine for maxillary buccal infiltrations in patients with irreversible pulpitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: 133-136
-
Claffey E, Reader A, Nusstien J, Beck M, Weaver J. Anaesthetic efficacy of articaine for inferior alveolar blocks in patients with irreversible pulpitis. J Endod 2004; 30:568-571
-
Kanaa MD, Whitworth JM, Corbett IP, Meechan JG. Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. Int Endod J 2009; 42: 238-246
-
Moore PA, Boynes SG, Hersh EV, De Rossi SS, Sollecito TP, Goodson JM, Leonel JS, Flores C, Peterson C, Hutcheson M. The anesthetic efficacy of 4% articaine 1:200,000 epinephrine: two controlled clinical trials. J Am Dent Assoc 2006; 137:1,572-1,581
-
Septodont Inc Septocaine(articaine hydrochloride 4% with epinephrine 1,100,000) injection prescribing formulation. Manufacturer’s Drug Information Leaflet.
-
Jung IY, Kim JH, Kim ES, Lee CY, Lee SJ. An evaluation of buccal infiltrations and inferior alveolar nerve blocks in pulpal anesthesia for mandibular first molars. J Endod 2008; 34: 11-13
-
Corbett IP, Kanaa MD, Whitworth JM, meechan JG. Articaine infiltration for anesthesia of mandibular first molars. J Endod 2008; 34: 514-518
-
Garisto GA, Gaffen AS, Lawrence HP, Tenenbaum HC, Haas DA. Occurrence of paresthesia after dental local anesthetic administration in the United States. J Am Dent Assoc. 2010; 141:836-844
-
Hillerup S, Jensen RH, Ersboll BK. Trigeminal nerve injury associated with injection of local anesthetics: needle lesion of neurotoxicity. J Am Dent Assoc. 2011;142:531-539
-
Robertson D, Nusstein J, Reader A, Beck M, Mc Cartney M. The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth. J Am Dent Assoc. 2007; 138:1104-1112
-
Haas DA, Lennon D. A 21 year retrospective study of reports of paresthesia following local anesthetic administration. J Can Dent Assoc 1995;61:319-330
-
Werdehausen WR, Fazeli S, Braun S, Hermanns H, Essman F, Hollman MW, Bauer I, Stevens MF. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth 2009; 103: 711-718
-
Haas DA. Localized complications from local anesthesia. J Calif Dent Assoc 1998; 26:677-682
-
Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 2004; 24:743-749
-
Pogrel MA, Schmidt BL, Sambajon V, Jordan RC. Lingual nerve damage due to inferior alveolar nerve blocks: a possible explanation. J Am Dent Assoc 2003; 134: 195-199
-
Stacey GC, Hajjar G. Barbed needle and inexplicable paraesthesias and trismus after dental regional anaesthesia. Oral Surg Oral Med Oral Pathol 1994; 77:585-588
-
Penarrocha-Diago M, Sanchis- Bielsa JM. Opthalmologic complications after intraoral local anesthesia with articaine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 90:21-24
-
Malanin K, Kalimo K. Hypersensitivity to the local anesthetic articaine hydrochloride. Anesth Prog 1995; 42:144-145
-
Torrente-Castells E, Gargallo- Albiol J, Rodriguez- Baeza A, Berini-Aytes L, Gay- Escoda C. Necrosis of the skin of the chin: a possible complication of inferior alveolar nerve block injection. J Am Dent Assoc 2008; 139:1,625-1,630
-
Elad S, Admon D, Kedmi M, Naveh E, Benzki E, Ayalon S, Tuchband A, Lutan H, Kaufman E. The cardiovascular effect of local anesthesia with Articaine plus 1:200,000 adrenaline versus Lidocaine plus 1:100,000 adrenalin in medically compromised cardiac patients: a prospective randomized double blinded study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105:725 -730
-
Oertel R, Rahn R, Kirch W. Clinical pharmacokinetics of articaine. Clin Pharmacokinet 1997; 33; 417-425
-
Becker DE, Reed KL. Local Anesthetics: Review of Pharmacological Considerations. Anesth Prog. 2012 Summer; 59(2): 90-102
-
Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: Mc Graw-Hill Companies Inc; 2011
-
Dionne RA, Goldstein DS, Wirdzek PR. Effects of diazepam premedication and epinephrine-containing local anesthetic on cardiovascular and catecholamine responses to oral surgery. Anesth Analg. 1984; 63:640-646
-
Hersh EV, Giannakopoulos H, Levin LM et al. The pharmacokinetics and cardiovascular effects of high-dose articaine with 1:100,000 and 1:200,000 epinephrine. J Am Dent Assoc. 2006;137:1562-1571
-
Schecter E, Wilson MF and Kong, YS, Physiologic responses to epinephrine infusion: the basis for a new stress test for coronary artery disease. Am Heart J 105:554-60, 1983
-
Dimsdale JE, Moss J. Plasma catecholamines in stress and exercise. J Am Med Assoc 1980; 243:340-2
-
Gandy W. Severe epinephrine-propranolol interaction. Ann Emer Med. 1989;18:98-99
-
Malamed SF, Handbook of Dental Anesthesia, Elsevier Mosby, St Louis Mo, USA, 5th edition, 2004
-
Doman S. An audit of the use of intra-septal local anesthesia in a dental practice in the South of England. Prim Dent Care 2011; 18: 143-147
-
Godzieba A, Smektala, Jedrzejewski M, Sporniak-Tutak K. Clinical Assessment of the safe use local anaesthesia with vasoconstrictor agents in cardiovascular compromised patients: A systematic review. Med Sci Monit. 2014; 20: 393-398
-
Tallman RD, Rosenblatt RM, Weaver JM, Wang YL. Verapamil increases the toxicity of local anesthetics. J. Clin Pharmacol 1988 Apr; 28(4): 317-21
-
Adsan H, Tulunay M, Onaran O. The effects of verapamil and nimodipine on bupivacaine-induced cardiotoxicity in rats: as in vivo and in vitro study. Anesth Anal 1998 April; 86(4): 818-24
-
Becker DE. Adverse drug interactions. Anesth Prog. 2011; 58:31-41
-
Foster CA, Aston SJ. Propranolol-epinephrine interaction: a potential disaster. Plast Reconstr Surg. 1983; 72: 74-78
-
Struthers AD, Reid JL, Whitesmith R, Rodger JC. Effect of intravenous adrenaline on electrocardiogram, blood pressure and serum potassium. Br Heart J 1982; 49: 90-93
-
Meechan JG, Rawlins MD. The effect of adrenaline in lignocaine anaesthetic solutions on plasma potassium in healthy volunteers. Eur J Clin Pharmacol 1987; 32: 81-83
-
Mimram A, Ribstein J, Sissman J. Effects of calcium channel blockers on adrenaline- induced hypokalaemia. Drugs 1993; 46(Suppl 2): 103-107
-
Peruse R, Goulet JP, Turcotte JY. Contraindications to vasoconstrictors in dentistry: Part 1, cardiovascular diseases. Oral Surg Oral Med Oral Pathol 1992, 74:679-86
-
Little JW, Falace DA et al. Dental Management of the Medically Compromised Patients, 5th ed. Mosby – Year Book, St Louis, 1997
-
Becker DC, Drug interactions in dental practice: a summary of facts and controversies. Compend Cont Educ Dent 1994, 15:1228-44
-
Muzyka BC. Atrial fibrillation and its relationship to dental care. J Am Dent Assoc 1999, 130: 1080-5
-
United States Department of Health and Human Services: Warning on Prescription Drugs Containing Sulfites, FDA Drug Bull, 17: 2-3, 1987
-
Seng GF, Gay BJ. Dangers of sulfites in dental local anesthetic solutions: warning and recommendations. J Am Dent Assoc 1986, 113: 769-70
-
Bush RK, Taylor SL et al. Prevalence of sensitivity agents in asthmatic patients. Am J Med 1986, 81:816-20
-
Perusse R, Goulet JP, Turcotte JY. Contraindications to vasoconstrictors in dentistry: Part II, hyperthyroidism, diabetes, sulfite sensitivity,cortico-dependent asthma and pheochromocytoma. O Surg O Med O Path 1992, 74: 687-91
-
Demas PN, JR Mc Clain. Hepatitis: implications for dental care. Oral Surg Oral Med Oral Pathol 88(1):2-4, 1999
-
Yagiela JA. Adverse drug interactions in dental practice: interactions associated with vasoconstrictors. J Am Dent Assoc 1999, 130:701-9
-
Goulet JP, Perusse R, Turcotte JY. Contraindications to vasoconstrictors in dentistry: Part III, pharmacologic interactions. Oral Surg Oral Med Oral Pathol 1992 74:692-7
-
Greenwood I, Heylen R, Zakrzewska JM. Anti-retroviral drugs – implications for dental prescribing. Br Dent J 1998; 184:478-482
-
Friedlander AH, Mahler M, Norman KM, Ettinger RL. Parkinson disease: systemic and orofacial manifestations, medical and dental management. J Am Dent Assoc 2009 Jun; 140(6): 658-69
-
Moore PA, Hersh EV, Papas AS et al. Pharmacokinetics of lidocaine with epinephrine following local anesthesia reversal with phentolamine mesylate. Anesth Prog. 2008; 55:40-48
There is no doubt that dentistry is a high-stress profession. Recent studies have reported that dental teams are subject to a variety of stress-related physical and emotional problems 1,2,3,4. These include heart disease, high blood pressure, adrenal fatigue, alcoholism, insomnia, depression and anxiety 5. Stress can be defined as ‘‘an adverse reaction that people have to excessive pressure or other types of demand placed on them’’.
Research suggests that the top five stressors in dentistry include running behind schedule, causing pain to our patients, heavy workloads, patient management and the treatment of anxious patients 2. As well as these factors, we have litigation on the increase 6 (the UK has now taken over the US with regards to litigation cases), and the GDC’s Fitness to Practise committees to worry about. When you take all of these factors into account, it is unsurprising that stress levels in dentistry are soaring to dangerously high levels. Yet, there is a lack of proactive measures being taken within our working environment and teams to address this issue of stress.
The signs of stress in an individual include feeling tense, feelings of anger and frustration, worry and anxiety, lack of concentration at home and at work, impaired sleep, depression, lack of interest in hobbies, poor appetite, comfort eating, increased consumption of alcohol and other stimulants to help to cope with the emotional impacts of stress. This can manifest within the dental team in many ways which include conflict within the team, absenteeism, a high turnover rate in staff, low morale, increased complaints, poor performance while at work and at times a lack of care in the standard of treatment provided. It is not only in the workplace that the effects of stress can manifest. They can also be present in the home and can cause problems with personal relationships. So, as there is no doubt that dentistry is a high-stress profession, what steps can we take within the dental team to prevent this?
We are regularly given advice on how to lead healthy lives, but sometimes we are so caught up in the stresses of life and work that we don’t know how or where to begin. I frequently hear colleagues suffering with stress say that they do not have time to fit exercise and attending the gym into their life due to their workload, commuting and their family commitments. It is only when they reach near-breaking point with their stress levels that they consider introducing some form of stress management into their life. I prefer a more preventive approach with stress involving a combination of regular physical exercise and well being activities such as yoga or tai chi.
I have been practising yoga for more than 15 years; initially it was the only type of exercise that I felt completely relaxed afterwards. I could go into a yoga session completely wired after a stressful day and come out the class an hour later in a blissful state. After a while I started to realise, though, that this blissful state would only last for a few hours after the yoga class or if I was lucky maybe 24 hours, and then the stress levels would rise again. What I realised was that if I made time to meditate every day in between the yoga classes I could return to the relaxed state that I had experienced quite easily to keep me going to my next class.
Meditation is an easy stress-management tool, which can be incorporated into your daily life very easily. When an individual is stressed, it is the sympathetic nervous branch of the autonomic nervous system that becomes overloaded. This is our ‘fight or flight’ response. The sympathetic nervous system (SNS) stimulates the adrenal glands when we are in flight or flight mode, releasing adrenaline and noradrenaline. This increases our heart rate, our blood pressure and our breathing rate. Once activated it can take anything from 20-60 minutes to return to pre-stimulation rates. By meditating and focusing on our breath we can turn off the SNS and switch on the parasympathetic nervous system (PNS), which is known as the ‘rest and digest’ response. When the PNS is activated, our heart rate drops, our blood pressure falls, our muscles relax and our breathing slows and deepens.
A practice of daily meditation can make our minds calm and peaceful, allowing us to take a step back from the hectic lives we are all leading. The more we incorporate it into our daily lives the more peaceful our minds become, the less stress our bodies have to cope with and we become happier in both our professional and personal lives.
Meditation is not a complicated practice; all we basically need to do is focus our minds. It can be done anywhere and at anytime, and the results can be seen very quickly by the individual. For me, I find that spending my last 15 minutes of my lunch hour sitting on my dental chair meditating is the time I can fit my daily meditation routine in. Of course, in order to be able to achieve this I have to be organised with taking my lunch to work with me everyday, either preparing my lunch the night before or purchasing it on the way to work in order to give me the time during my lunch break to meditate. When I first started to do this at lunchtime, no one actually knew what I was doing in my surgery. I think everyone thought I was sat relaxing listening to music on my iPhone!
How to begin meditating
The practice is a very simple one. I would suggest you find a quiet space and sit down. You can sit normally on a chair or sit on your dental chair, being comfortable with a straight back is the main aim. Keeping your eyes open initially, bring your focus onto your breathing. I like to do a three-part breath, that is taught in yoga, to initially calm my breathing down. I breath into my stomach, rise the breath into my chest and feel my back expand and then feel my collar bones rise. To exhale I lower the collar bones, deflate my chest and pull in my stomach gently squeezing the last of the breath. I repeat this for a few minutes, then I close my eyes.
Depending on how my mind is on the day will determine what I focus on during my meditation. My mind will vary from day to day like any other mind. What I have found that really helps me to deal with all of my thoughts is to accept and acknowledge that, once my meditation is over, I will be able to get to them, and that the time I allocate for meditation is just for that. Everything will be still be there in 15 minutes when I start work again. Meditation is the process of getting to know our mind and understand it. The trick is not to identify with your thoughts and give them any energy or focus, just observe them.
Once my eyes are closed I continue with my breathing and do a mental scan of my body to see where any tension may be. Working my way from my head down to my toes I relax any tension that I have in my body, and then return my focus to my breath. Any thoughts that come into my head I let come and go, observing them without feeding into them. At this point my mind starts to settle fully and becomes peaceful.
This is the parasympathetic nervous system switching on and starting to do all of the good work in getting you back to feeling human again. I like to stay in this headspace with my eyes closed for as long as I can, at least 10 minutes. I use an app on my phone to time this, which really helped in the beginning as it stopped me worrying about how long I was sitting there for.
When my mediation time is up, I open my eyes and carry on with my working day. It is now part of my daily working routine, which I look forward to each day.
Guided meditation
If you find that the breathing meditation does not work for you in the beginning you can try a guided mediation. This is a when you are guided into a relaxed state by a teacher or trained practitioner. There are many of these available in app form and also free on YouTube.
Mantra meditation
This is the practice of repeating a mantra over and over in your mind during your meditation. It is a really good method of meditation for beginners as it really helps to focus your mind on the words that you are saying, giving a distraction from your thoughts. The mantra can be anything you want, from a positive affirmation of ‘I change my thoughts, I change my world’ to using a Hindu mantra, an example being a simple ‘Om’ or ‘Om Namah Shivaya’ or just a simple phrase or word like relax or peace.
Yoga and meditation
You gain the most benefit from your meditation when combining it with a yoga practice. Yoga has the ability to trigger both the SNS and the PNS, and is designed, when practised in its traditional form, to prepare you for meditation. A good rounded yoga class will trigger the SNS at the beginning of the class with sun salutations and more challenging postures then introduce postures that trigger the PNS such as seated forward bending postures and shoulder stands (these can be easily done by beginners by placing their legs up against a wall with their back staying flat on the floor), before taking you into yoga nidra which is the deep relaxation at the end of a yoga class, which stimulates the PNS further. This is followed by some breathing exercises, or pranayama as it is called in yoga, to help prepare your mind further for the meditation at the end of the class, which again stimulates the PNS further.
Apps to help with meditation
There are many apps available either free or at a small cost to help you with your meditation. I find that these can be really helpful with introducing a new habit or routine. As with any form of behaviour change it is forming a good habit and getting into a good routine that will ensure you keep the daily practise going. The apps monitor your progress and help with the formation of new habits with different methods of reinforcement.
- Headspace (Fig 1)
Headspace is an app that has 10 free introductory sessions before requiring you to subscribe. It is available from the app store and is a good way to introduce meditation into your daily routine. It records your progress with each session building on the last. If you subscribe, there are sessions available to help with many different topics from depression to over eating. - Insight (Fig 2)
The Insight timer is another app available from the app store. It is a timer for your meditation where you can determine the length of time you would like to meditate for. It is also has a great resource of guided meditations available free on topics including an introduction to meditation, morning meditations, sleep meditations, relaxation, mindfulness, self love and compassion and a collection of talks and podcasts. - Oprah and Deepak’s 21-day meditation (Fig 3)
Oprah Winfrey has teamed up with Deepak Chopra, introducing a free 21-day mediation course at different times throughout the year. There are 21 daily downloads of meditation and wisdom from Deepak and Oprah, with each day building on the previous day’s session. If you miss the sign-up for this programme you can purchase it from their website www.chopracentremeditation.com
Alternatively you can visit Deepak’s own website – www.deepakchopra.com – which is a great resource for information on meditation including many free downloads. - Breathe (Fig 4)
This app monitors your progress and also has a range of free guided meditations. It is designed to reinforce behavioural change and habits with rewarding you with different stickers when you have completed any of the goals that are set for you by the app.
- Figure 2 – Insight timer app
- Figure 3 – Oprah and Deepak’s 21-day meditation app
- Figure 4 – Breathe app
Conclusion
Dentistry is a high-stress profession, not just for the dentist but also for each member of the team. If we do not manage our stress levels effectively, eventually we will become demotivated, burn ourselves out and put ourselves at risk of stress-related illnesses. Forming a good habit of taking a time out of 10-15 minutes each day to relax and refocus can help to reduce stress levels of all members of the team.
About the author
Morag has been involved in the dental industry for 25 years. She is dually qualified, as a hygienist and a therapist, with a primary focus on delivering high-quality dental care in private practice. She has a special interest in adult periodontics, peri-implantitis and composite restorations.
An advocate of lifelong learning, she has recently completed the BSc (Hons) course in Dental Studies at UCLAN. Prior to relocating to Gibraltar, Morag ran a study club for hygienists and therapists in the north of Scotland, delivering education to local colleagues. Morag is also a consultant for the Swiss dental instrument company Deppeler and a tutor for Aspiradent, a postgraduate teaching company.
Working as lead hygienist therapist at Fergus & Glover, Morag led the team to win the ‘Best Preventive Practice’ in the UK at the DH&T awards 2012, and has been shortlisted for both Hygienist of the Year at the DH&T awards and DCP of the Year at the Scottish Dentistry awards.
References
1. R. Rada, C. Johnson-Leong. Stress, burnout, anxiety and depression among dentists. The Journal of the American Dental Association. June 2004, Vol. 135(6): 788-794, doi: 10.14219
2. J. Ayatollahi, F. Ayatollahi, M. Owlia. Occupational hazards to dental staff. Dental Research Journal. Jan-Mar 2012:9(1):2-7
3. A. Puriene, V Janulyte, M. Musteikyte, r. Bendinskaite. General health of dentists – Literature review. Baltic Dental and Maxillofacial Journal. 2007, 9:10-20
4. H. Myers, L. Myers. ‘It’s difficult being a dentist’: stress and health in the general dental practitioner. Br Dent J. 2004. Jul 24;197(2):89-93
5. J. Lang. Stress in dentistry – it could kill you! Oral health. Available from: http://www.oralhealthgroup.com/features/stress-indentistry-could-kill-you/ .(Accessed June 2016)
6. J. Hyde. ‘Unprecedented’ clinical negligence claims as PI firms migrate. The Law Society Gazette. July 2014
Depending on what you read, there are various criteria proposed for assessment of endodontic outcome and these differ depending on whether a surgical or non-surgical approach has been used. The European Society of Endodontology proposes the following definition of non-surgical root canal treatment (NSRCT) outcome:
- Healed: Absence of clinical signs and symptoms and a return to normal architecture of the periodontal ligament radiographically.
- Healing: Absence of clinical signs and symptoms and reduction in size of the periapical lesion.
- Failure: Presence of clinical signs/symptoms and/or no reduction in lesion size, increase in lesion size or emergence of a new periapical lesion.
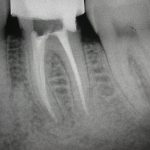
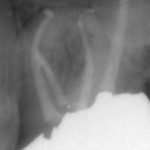
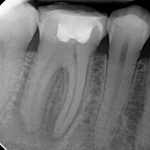
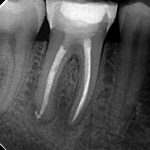
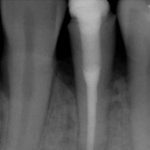
From the literature, it would seem that success rates of NSRCT are relatively high depending on the criteria used yet often teeth exhibiting reduced lesion size are hard to classify. Although Orstavik (1996) highlighted that teeth showing these radiographic signs of healing at 12 month review, continue to heal in almost 90 per cent of cases (Figs 1a and b). For those dubious cases it is important to arrange follow up for anything up to four years and not condemn a tooth to failure too early (Strindberg 1956).
Does survival count?
The stringent outcome criteria outlined above have been used to assess NSRCT for decades. More recently, however, survival rates have been mentioned in the endodontic literature (Salehrabi 2004).
This has partly been driven by the endodontic discipline in order to level the playing field between endodontics and implants. Much of the implant literature uses this less stringent definition of treatment outcome, with survival rates in excess of 90 per cent being reported. The reality is that it is very difficult to compare the two treatment modalities and unnecessary also as both have a place in daily practice life. Direct comparisons, although shining a favourable light on endodontics (Doyle 2006) are also confusing and probably best avoided.
More recently, the term functionality has appeared in the endodontic literature and this may be more relevant to practice. Friedman (2004) described this as a tooth presenting with an absence of clinical signs/symptoms but with evidence of pathosis radiographically. How often do we see patients who, when faced with the prospect of dismantling a tooth which has been in function for decades and exposing themselves to the risk of it being unrestorable, choose to continue to monitor the situation instead? This may pose problems later in terms of potential flare up and possible systemic impacts of oral infection although the evidence supporting the later in the endodontic literature is scarce.
Are cracked teeth doomed to failure?
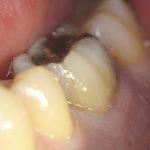
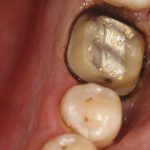
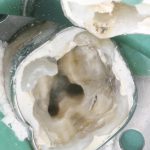
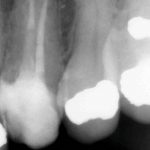
Diagnosis of and prognostication in relation to cracked teeth is difficult. Where does the crack end? How likely is it to progress? Will it affect longevity? All of these questions spring to mind when we consider this clinical scenario.
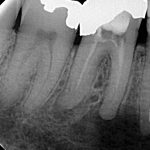
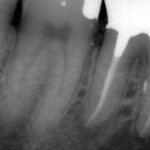
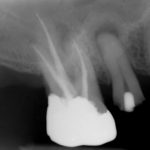
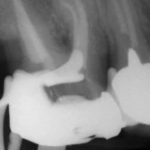
Patients presenting with symptoms of a crack in a vital tooth should always be investigated thoroughly and this often involves removal of the direct restoration, visualisation of the crack under magnification and illumination and possibly a period of stabilisation with a stainless steel orthodontic band. In the cases of root filled teeth, again the tooth should be dismantled and the crack visualized in order to attempt to assess its severity (Figs 2a-c). Care should also be taken to identify and assess any soft tissue signs such as multiple draining sinuses or deep narrow isolated probing defects adjacent to heavily restored root filled teeth. Cracks can often be very difficult to diagnose radiographically unless there is frank separation of the tooth fragments although a characteristic J-shaped radiolucency may indicate a split tooth.
The American Association of Endodontists (AAE) have attempted to classify cracked teeth according to severity, ranging from an enamel infraction up to a split tooth and this is helpful in terms of reaching a diagnosis and labelling these teeth. It does not, however, offer much help in terms of deciding on whether to treat or extract these teeth. Traditionally, it was felt that the presence of a crack on the floor of the pulp chamber often precluded endodontic treatment, but more recent outcome studies have highlighted the importance of the presence of a deep probing defect associated with the tooth as a negative prognostic factor (Tan et al. 2006, Kang et al. 2016) while others have found that extension on to the pulpal floor resulted in greater tooth loss (Sim et al. 2016). So, that just seemingly adds to the confusion.
Most importantly, the patient needs to be made aware of the presence of a crack and its potential effect on outcome. Ultimately, it is the patient who decides, although I would personally caution against attempting to save teeth with extensive cracks due to the detrimental effect this would have on the surrounding bone should the crack propagate and the subsequent effect on the bony site for implant placement.
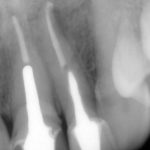
Does rubber dam actually matter?
Although the use of rubber dam is widely recommended during endodontic treatment, two things become clear when the evidence is examined more closely:
- Rubber dam is not routinely used during treatment. According to Whitworth et al. (2000), 60 per cent of dentists never use it, with factors such as patients’ perceptions, time to apply and a lack of training being cited as obstacles.
- There is actually very little in the literature which highlights the positive impact rubber dam use can have on the outcome of endodontic treatment, with only van Nieuwenhuysen et al. (1994) and Lin et al. (2014) showing a positive association.
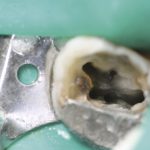
However, this should not mean that we do not recognise the multiple benefits of rubber dam use during endodontic treatment, namely retraction of soft tissues, better visualisation and protection of the airway among them. The reality is that rubber dam makes our job easier and that makes a difference (Fig 3).
What effect do missed canals have?
Uninstrumented and unfilled root canal anatomy can clearly have an effect on endodontic outcome, and nowhere is this more evident than in the case of the second mesiobuccal canal or MB2 in maxillary molars.
The key to successful identification of root canal anatomy lies in the design and execution of our access cavity. Large, overblown access cavities, incorrect positioning and small contracted cavities lend themselves to problems later. Magnification and illumination are also key with Kulild & Peters showing that almost 10 per cent of MB2 canals were not identifiable without the aid of a dental operating microscope (DOM). They also highlighted the fact that the MB2 is present in excess of 90 per cent of the time (Fig 4a-d).
The effect of missed canals on outcome may be a function of canal configuration in that canals joining before the apex are less likely to have a negative effect. Their presence may also play a strong role in decision-making in failed cases in terms of choosing a surgical or non-surgical approach. Wolcott et al. (2005) discussed the impact of a missed MB2 on prognosis and highlighted its increased incidence in non-surgical re-treatment cases.
- Figures 1a and 1b Pre-operative and six-month review radiographs following root canal re-treatment showing signs of healing
- Figure 1b
- Figure 2a – Photograph showing crack at MB aspect of tooth 36
- Figure 2b Radiograph following endodontic treatment
- Figure 2c Tooth prepared subsequently for full coverage restoration
- Figure 3 Maxillary molar isolated under rubber dam (note stabilisation with orthodontic band and Oraseal used to fill deficiencies in dam)
- Figures 4a-c Radiographs above showing MB anatomy following obturation in both primary and re-treatment cases
- Figure 4b
- Figure 4c
- Figure 4d Photo showing position of MB2 on floor of pulp chamber
- Figure 5a Periapical radiographs showing satisfactory obturation and almost complete resolution of lesion at six-month review
- Figure 5b Periapical radiographs showing satisfactory obturation and almost complete resolution of lesion at six-month review
- Figures 6a-c Radiographs showing teeth restored with fiber post and composite core, direct composite core and cast post and core
- Figure 6b
- Figure 6c
Which factors actually affect the outcome?
Outcome studies have highlighted many factors that affect endodontic outcome with the length of the root filling, its density and the quality of the coronal restoration being common to many of them. Ng et al. (2011) cited 13 factors that can affect the outcome of both primary and secondary NSRCT. Interestingly, many of these factors are related to our treatment of root canal infection and obturation, of course, is merely a surrogate measure of what went before. The key to successful treatment lies in the adherence to strict biological principles (isolation, hypochlorite use, patency, dense obturation to length) and the execution of this clinically (Fig 5).
How important is the coronal restoration?
The role of the coronal restoration is also important. Many papers have discussed this with some proposing the restoration as a more important prognostic factor (Ray and Trope 1995), others emphasising the importance of the restoration (Tronstad 2000) and others wisely stating the importance of both (Hommez et al. 2002).
Ng et al. (2011) describes a number of ‘restorative factors’ that may affect the outcome:
- Presence of temporary vs. cast restoration
- Terminal location of tooth
- Presence of cast post and core
- Missing proximal contacts.
It has been routinely accepted that indirect cuspal coverage for posterior root filled teeth with breached marginal ridges constitutes best practice, although Sequeira-Byron et al. (2015) in their Cochrane review, have questioned the evidence base for this although they stop short of not recommending cuspal coverage clinically.
The issue of cast/metal versus fiber posts is also one which is raised regularly (Figs 6a-c). The issues of root fracture and dentine removal are reported as disadvantages of the former while issues with bonding to root canal dentine are cited by those opposing the use of the latter. Theodosopoulou and Chochlidakis (2009) in their systematic review on the subject reported that carbon fiber posts perform better than precious metal cast posts although there are numerous other sources which are not in agreement with this. The reality is that decisions should be made on a case by case basis based on both canal anatomy and remaining dentine distribution and thickness.
How often should I review the patient?
Although Orstavik found radiographic signs of healing within three weeks post-treatment, it is almost universally accepted that a minimum period of six months should elapse before review is considered. The ESE recommend clinical and radiographic review at one year and continued follow up for a period of four years depending on the initial response.
Fristad et al. reported ‘late successes’ with radiographic signs of healing being evident up to 27 years after treatment. They also caution against labeling radiographic overfills with persistent radiolucencies as failures as delayed healing is often seen in these cases and they are best monitored.
Clinically, we do also sometimes encounter patients who appear to have inexplicable symptoms of discomfort or awareness of a root filled tooth, even in the presence of radiographic signs of healing. Polycarpou et al. (2005) reported the prevalence of persistent pain following successful root canal treatment to be as high as 12 per cent, with a number of factors influencing this including pre-operative tenderness to percussion, a history of chronic pain and presence of pre-operative pain associated with the tooth. We should possibly remember this when consenting patients in certain cases prior to treatment.
Conclusions
In conclusion, the factors affecting the outcome of endodontic treatment, our role in achieving it and the required follow up period and protocol are clear. Unfortunately, despite all of this as Marton and Kiss (2000) outlined in their review paper, root canal treatment is merely a permissive factor for healing and so we do not have direct control over it. We can only try our best and hope that it is good enough.
References
Doyle, Scott L, et al. “Retrospective cross sectional comparison of initial nonsurgical endodontic treatment and single-tooth implants.” Journal of Endodontics 32.9 (2006): 822-827.
Friedman, Shimon, and Chaim Mor. “The success of endodontic therapy healing and functionality.” CDA J 32.6 (2004): 493-503.
Fristad, I, O Molven, and A Halse. “Nonsurgically retreated root filled teeth–radiographic findings after 20–27 years.” International Endodontic Journal 37.1 (2004): 12-18.
Hommez, GMG, CRM Coppens, and RJG De Moor. “Periapical health related to the quality of coronal restorations and root fillings.” International Endodontic Journal 35.8 (2002): 680-689.
Kang, Sung Hyun, Bom Sahn Kim, and Yemi Kim. “Cracked Teeth: Distribution, Characteristics, and Survival after Root Canal Treatment.” Journal of Endodontics 42.4 (2016): 557-562.
Kulid, James C, and Donald D Peters. “Incidence and configuration of canal systems in the mesiobuccal root of maxillary first and second molars.” Journal of Endodontics 16.7 (1990): 311-317.
Lin, Hui-Ching, et al. “Use of rubber dams during root canal treatment in Taiwan.” Journal of the Formosan Medical Association 110.6 (2011): 397-400.
Marton, IJ, and C Kiss. “Protective and destructive immune reactions in apical periodontitis.” Oral Microbiology and Immunology 15.3 (2000): 139-150.
Ng, YL, V Mann, and K Gulabivala. “A prospective study of the factors affecting outcomes of non-surgical root canal treatment: part 2: tooth survival.” International Endodontic Journal 44.7 (2011): 610-625.
Ørstavik, D. “Time-course and risk analyses of the development and healing of chronic apical periodontitis in man.” International endodontic journal 29.3 (1996): 150-155.
Polycarpou, N, et al. “Prevalence of persistent pain after endodontic treatment and factors affecting its occurrence in cases with complete radiographic healing.” International Endodontic Journal 38.3 (2005): 169-178.
Ray, HA, and M Trope. “Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration.” International Endodontic Journal 28.1 (1995): 12-18.
Salehrabi, Robert, and Ilan Rotstein. “Endodontic treatment outcomes in a large patient population in the USA: an epidemiological study.” Journal of Endodontics 30.12 (2004): 846-850.
Sequeira-Byron P, Fedorowicz Z, Carter B, Nasser M, Alrowaili EF. Single crowns versus conventional fillings for the restoration of root-filled teeth. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No.: CD009109. DOI: 10.1002/14651858.CD009109.pub3
Sim, Irene GB, et al. “Decision Making for Retention of Endodontically Treated Posterior Cracked Teeth: A 5-year Follow-up Study.” Journal of Endodontics 42.2 (2016): 225-229.
Strindberg, Lars Z. The dependence of the results of pulp therapy on certain factors: an analytic study based on radiographic and clinical follow-up examinations. Vol. 12. Mauritzon, 1956.
Tan, L, et al. “Survival of root filled cracked teeth in a tertiary institution.” International Endodontic Journal 39.11 (2006): 886-889.
Theodosopoulou, Joanna N, and Konstantinos M Chochlidakis. “A systematic review of dowel (post) and core materials and systems.” Journal of Prosthodontics 18.6 (2009): 464-472.
Tronstad, L, et al. “Influence of coronal restorations on the periapical health of endodontically treated teeth.” Dental Traumatology 16.5 (2000): 218-221.
Van Nieuwenhuysen, JP, M Aouar, and William D’Hoore. “Retreatment or radiographic monitoring in endodontics.” International Endodontic Journal 27.2 (1994): 75-81.
Whitworth, JM, et al. “Use of rubber dam and irrigant selection in UK general dental practice.” International Endodontic Journal 33.5 (2000): 435-441.
Wolcott, James, et al. “A 5 yr clinical investigation of second mesiobuccal canals in endodontically treated and retreated maxillary molars.” Journal of Endodontics 31.4 (2005): 262-264.
About the author
Dr Robert Philpott, BDS MFDS MClinDent MRD (RCSEd), qualified from Cork Dental School in 2003 and completed his endodontic training at Eastman in London in 2009. He has worked as a specialist in endodontics in Ireland, London and Australia. He currently divides his time working as a consultant in endodontics at Edinburgh Dental Institute and in private practice at Edinburgh Dental Specialists.
Would you turn away more than half your patients because you don’t offer the treatment they need? That’s just what you could be doing. A recent survey 1 of 2,438 UK residents in co-habiting relationships found that 61 per cent of respondents in Scotland admitted that either they or their partner snored. Of all the regions and counties in the UK, Scotland came out top in terms of percentage of snorers in the population.
With training and minimal outlay on equipment, you could treat those of your patients who snore and market the treatment as a service to potential new patients. To assess the demand, simply add these questions to your routine medical health questionnaire:
Do you wake with a headache in the morning? Or do you or your partner snore?
Do you feel tired or sleepy during the day?
Sleep apnoea explained
Before I explain how to introduce Dental Sleep Medicine (DSM) in your practice, here’s some background information. Snoring is a sleep-related breathing disorder (SRBD), along with or without obstructive sleep apnoea (OSA).
Snoring and excessive daytime sleepiness are two cardinal symptoms of obstructive sleep apnoea. All apnoeacs are snorers but all snorers are not apnoeac. There is a distinction between central sleep apnoea (CSA) and OSA. CSA is the result of the sleeping person not breathing due to problems with the central respiratory drive that fails to transmit the appropriate signals between the brain’s respiratory centre and the respiratory muscles, which fail to act. OSA is the result of collapse of the unsupported airway due to disparity between the pressure within the airway versus that outside the airway. This leads to reduction of oxygen saturation in the blood and increase of carbon dioxide in the system due to failure to exhale it – hypoxia and hypercapnia.
CPAP and MRDs
Continuous Positive Airway Pressure (CPAP), where sleeping patients wear a face or nasal mask connected to a pump that produces a positive flow of air into the nasal passages to keep the airway open, is widely regarded as the most effective treatment. Surgery is usually an option of last resort.
However, although CPAP is very effective, many people can’t tolerate wearing the mask. This is where DSM comes in because the alternative, a custom-made intra-oral appliance or mandibular repositioning device (MRD), worn in the mouth at night can greatly reduce, if not completely eliminate, snoring. MRDs are primarily indicated for the treatment of simple, non-apnoeic snoring as well as for mild to moderate OSA when prescribed and monitored as part of a multidisciplinary team. I’ll write more about MRDs below.
Integrating DSM
The steps involved in becoming a practice that offers treatment for snoring are training, networking, continuous education and promotion. A dentist in the team needs appropriate training, they should network with local medical practitioners and specialists in respiratory medicine and ENT, the reception staff, hygienist and nurses must be educated in the practice’s new dental sleep medicine service and promote the service widely.
- Fig 1 The SomnoDent
- Fig 2 ResMed Narval CC
- Fig 3 George Gauges. Photo courtesy of Eurodontic Ltd
Training
Knowledge of the disease is essential for introducing DSM into your practice. A wider knowledge of other sleep disorders is useful but not essential. The British Society of Dental Sleep Medicine (BSDSM) of which I am president, trains dentists to screen, assess for SRBDs and follow a documented pathway of practice to provide the most appropriate treatment for the patient. Most BSDSM courses are of one-day duration and include hands-on experience with MRDs. You will learn what equipment you need (not very much) and meet people who have introduced DSM into their practice. Dental technicians also attend these courses to learn about MRDs so you may wish to team up with someone from the laboratory you use. Go to www.dentalsleepmed.org.uk for more information and details of BSDSM courses.
The treatment of snoring and OSA does not fall within the scope of dentistry. However, the professional indemnity insurers, DPL and DDU, each have a position statement for their members who provide DSM treatment who can seek medico-legal advice, if the member has documented evidence of having followed appropriate care and is duly qualified to provide such treatment.
Networking
While in the past it was often not keen to work with dentists, the medical profession – physicians and ENT consultants – is actively seeking qualified dentists to treat patients not suitable for or intolerant of CPAP. Also, GPs refer simple snorers and mild apnoeacs, who are not suitable nor eligible for secondary referral to a sleep centre, to dentists. You should introduce yourself to local medical practitioners and specialists in respiratory medicine and ENT.
Education
It’s important to disseminate information about your new DSM service to all your team members, including reception staff. When asked if there’s a treatment for snoring, they should answer with sufficient knowledge to encourage the person to make an appointment for an initial assessment. They also need to provide a broad guide to the likely costs involved.
You’ll need to decide on a fee scale for DSM. Allow at least an hour for a new patient’s first visit for a snoring and OSA assessment and possibly impressions for an MRD. Most practices have a standard initial assessment charge, then an overall fee for providing an MRD (each device will have a different cost) and initial follow-up visits for adjustment.
Promotion
Members of the BSDSM who are dentists with a special interest in snoring and sleep apnoea are listed on the ‘Find a dentist near you’ page of the BSDSM website. There are currently only four in Scotland so you will be entering a field where likely demand is much greater than supply. Nevertheless, you should promote your new service in all the usual ways, including adding (for example) ‘Do you snore?’ to your patient medical history form.
MRDs – an overview
Although there are several types of MRD and around 100 different ones on the market, they all share these principles: – to open the vertical dimension of the airway – there are different theories about the amount of opening vertical dimension (OVD) required and to position the mandible and tongue forward.
They must also satisfy the following criteria:
- Be made from biocompatible materials
- Be durable
- Have an acceptable neutral taste and smell
- Be adjustable
- Have good retention
- Have a track record of minimal side effects
- Have acceptable aesthetics
- Be cost effective – which may be countermanded by durability.
The materials used range across hard acrylic and soft thermoplastic to cobalt chrome and can affect the degree of customisation possible. Coupling mechanisms – used to connect the upper and lower splints – may be located in anterior or posterior segments. Some MRDs allow lateral and vertical mandibular movement, whereas others lock the mandible in position.
In summary, an MRD that functions correctly and is comfortable for the patient results from an accurate clinical assessment by the dentist combined with expertise and skill from the dental technician and close liaison between the two is vital.
Conclusions
Integrating DSM into a dental practice is straightforward, not expensive and not time consuming. In Scotland, even more so than other parts of the UK, there is a large cohort of potential patients. Globally, steady growth has been consistently forecast in the sleep apnoea devices market – a recent report 2 predicting a 7.7 per cent increase by 2020. What are you waiting for?
About the author
Dr Aditi Desai is President of the British Society of Dental Sleep Medicine (BSDSM). She has accreditation from the European Academy of Dental Sleep Medicine, serves on the Council of the Odontological and Sleep Section at the Royal Society of Medicine. Dr Desai consults from 76 Harley Street, and London Bridge Hospital.
References
- www.ergoflex.co.uk/blog/category/sleep-research/snoring-map-of-the-uk (accessed 20 January 2016)
- www.grandviewresearch.com/industry-analysis/sleep-apnea-devices-market (accessed 20 January 2016)
There is rising interest in and demand for adult orthodontics in the UK. While much of this demand is met by the specialist orthodontist body, there are a number of techniques marketed to general dental practitioners as being more acceptable to adult patients.
Whether a clear aligner system or a “short term orthodontics” system with limited objectives, the principle aims of these systems seem to be trying to minimise the duration and cosmetic impact of treatment, while perhaps accepting that significant objectives, such as occlusal correction, root paralleling or torque control are sacrificed.
Lingual orthodontics has the potential to offer a truly aesthetic solution to both adult and adolescent orthodontic treatments, without making unnecessary compromises to the clinical outcomes. Surprisingly, the uptake in the UK remains marginal. In 2014, of 1,357 GDC registered specialists, approximately 750 had undergone training or certification in lingual appliance systems. However, less than one third of these trained specialists carried out any lingual treatments, with only around 3,000 cases treated annually. It would seem that there are significant barriers to patients being offered lingual treatment. This article aims to examine these barriers and see how they can be overcome.
Contemporary, fully customised, lingual appliances start with the end point in mind. There are many systems available. The most commonly-used system worldwide is Incognito (3M healthcare) developed by Dr Weichmann 1. After taking accurate silicone impressions or intra-oral scans, a physical or virtual set up of the idealised occlusion is produced. Following this, brackets are digitally designed to be as close fitting to the lingual tooth surface as possible.
Wires are then custom bent by a 3D bending robot to connect the brackets that, when fully aligned, will precisely deliver the set up occlusion. Accurate bracket placement is crucial and indirect bonding trays must be used. Excellent precision of the final archwire to bracket contact is essential to ensure precise delivery of the setup (Fig 1).
Patient adaptation
Early lingual appliances, such as the Ormco 7th Generation (Fig 2) bracket were large and the indirect bonding customisation often resulted in significant protrusion from the lingual aspect of the tooth, encroaching upon tongue space. Patients would often report slow adaptation including difficulty with speech, tongue discomfort and eating 2. Over the last two decades, manufacturers have produced smaller brackets and the advent of fully customised systems that minimise both bracket and wire protrusion towards the tongue have greatly reduced these problems 3.
Dental health
It would seem logical that placing brackets lingually would complicate oral hygiene, increasing the risk of periodontal problems and caries. When periodontal health has been monitored, it has been shown that lingual appliances do result in slightly increased plaque and bleeding indices 4 but that these changes are completely reversible with no long-term increase in the presence of periodontal pathogens 5.
When the incidence of caries and white spot lesions has been examined, the results are surprising. Compared with labial appliances, lingual appliances result in 4.8 times fewer lesions, which, when present, show up to 10 times less intensity of decalcification 6. Therefore, there is in fact a compelling argument to use lingual appliances to reduce the risk of iatrogenic enamel damage compared with conventional labial appliances.
Outcomes
A possible barrier to the use of lingual appliances is the perception that clinical outcomes may not be as good as with labial appliances. Indeed, for any dentist to use a newer clinical approach, there is an ethical obligation to ensure that it will provide an outcome which is at least as good as that achieved by other proven, tried and tested appliances and techniques. There is evidence that well managed lingual appliances fulfil this requirement.
In a prospective comparison with matched labial and lingual cases, no difference was found in quality of outcomes, treatment time or apical root resorption between the two appliance groups 7. Further evidence on the potential accuracy of contemporary lingual appliances is in how closely treatment outcomes match the predicted set up occlusion. In a study where digital scans of the final occlusion were superimposed on the predicted setup, it was shown that 94 per cent of the planned movements were achieved 8. By comparison, the accuracy of Invisalign under similar investigations varied from 41 per cent to 59 per cent 9,10. It seems that lingual appliances have the potential to aesthetically deliver outcomes that are at least as good as those that we can achieve with conventional labial comprehensive orthodontic treatment.
- Figure 1a
- Figure 1b
- Figure 1c
- Figure 1d
- Figure 1e
Biomechanics
By applying forces to the lingual aspect of the teeth, the actions, reactions and moments are different to what we see with labial appliances. As the point of force application is distant from the labial surface of the tooth, we are effectively carrying out orthodontics at the end of a broom-handle! Accuracy and precision is essential. Finite element studies have shown that there is a greater tendency towards lingual tipping, vertical bowing of the occlusal planes and torque loss upon retraction 11,12. Various appliance modifications are made to counter these effects, such as optimal bracket and wire precision, close fit of bracket to tooth surface and ribbon-wise rather than edgewise archwires.
Technique sensitivity
This is perhaps the greatest hurdle to the widespread use of lingual appliances. The access to the lingual aspect of the teeth is certainly more difficult than with labial appliances or aligner systems. Contemporary brackets are small to ensure comfort and there is a lack of familiarity for the new lingual operator. The manufactures are aware of this issue and all systems are constantly evolving features and ligation techniques to make the life of a lingual orthodontist easier. It has been shown that self-ligating lingual brackets (Fig 3) will make life easier for the novice clinician, but that this benefit over a ligature-based system decreases as experience is gained 13. As with other fields of clinical practice, skills increase exponentially with experience and focused learning.
Digital planning
There has been much debate in the orthodontic world on where we should plan to position teeth. Decades of research and retrospective analysis have suggested that maintaining initial archform will minimise the risk of relapse 14. The introduction of novel treatment philosophies based on avoiding extractions 15 have optimistically hypothesised that there are no limits to where we can place teeth and that the alveolar bone will develop to accommodate any positions that we choose to place teeth.
However, met-analysis of clinical studies 16 and CBCT studies 17 have shown that the alveolus has limited adaptive potential. Basically, we must place the roots within the pre-treatment trough of alveolar bone if we are to maximise stability and minimise the risk of gingival recessions.
The digital planning platforms, such as 3M Treatment Management Portal or Sure Smile software allow the clinician to precisely plan where the final occlusion and tooth position will be, with reference to pre-treatment anatomy, considering aesthetics, stability and respecting constraints of the supporting tissues. With the precision of customised lingual appliances, we have the ability to accurately deliver the planned final occlusion (Fig 4), improving our treatment outcomes and potentially reducing iatrogenic effects.
As technology develops to allow better integration with CBCT and digital photography for aesthetic analysis, the clinician’s ability to plan and deliver optimal outcomes will continue to be enhanced.
Conclusions
There is a danger in hoping that technology or smart marketing can solve all of our clinical problems. For a clinician to become truly competent in a technique, he must be prepared to invest effort and time in becoming familiar with the principles, mechanics and effects of an appliance in both the educational and clinical settings. For those prepared to do so, the rewards for our patients are apparent. Carefully managed lingual appliances with digital planning platforms now provide the clinician with the tools to plan and precisely deliver optimal orthodontic treatment (Fig 5).
About the author
Robbie Lawson is a partner in Edinburgh Orthodontics, a specialist practice committed to achieving optimal outcomes for all patients. He did his dental training at Dundee University, graduating with honours in 1990. He undertook his orthodontic specialty programme at the University of Wales, graduating with distinction in 1996. He gained his Membership in Orthodontics from the Royal College of Surgeons of Edinburgh, winning the William Houston gold medal. Since 1996, he has been in specialist practice in Edinburgh. He is past chairman of the Scottish Orthodontic Specialists Group, and is an examiner at the Royal College of Surgeons of Edinburgh.
He has had a special interest in lingual orthodontics since 1999, having used a wide range of lingual appliance systems over the years. He has published clinical and research papers and presents regularly nationally and internationally on fully customised aesthetic appliances. He is a member of the Worldwide Incognito Lingual Appliance Advisory Board.
- Figure 2
- Figure 3
- Figure 4
- Figure 5a
- Figure 5b
- Figure 5c
- Figure 5d
- Figure 5e
- Figure 5f
- Figure 5g
- Figure 5h
- Figure 5i
- Figure 5j
- Figure 5k
- Figure 5l
- Figure 5m
- Figure 5n
References
1. Wiechmann D, Rummel V, Thalheim A, Simon JS, Wiechmann L. Customized brackets and archwires for lingual orthodontic treatment. Am J Orthod Dentofacial Orthop. 2003 Nov;124(5):593-9
2. Caniklioglu C, Oztürk Y. Patient discomfort: a comparison between lingual and labial fixed appliances. Angle Orthod. 2005 Jan;75(1):86-91.
3. Hohoff A, Stamm T, Goder G, Sauerland C, Ehmer U, Seifert E. Comparison of 3 bonded lingual appliances by auditive analysis and subjective assessment. Am J Orthod Dentofacial Orthop. 2003 Dec;124(6):737-45.
4. Miethke RR, Vogt S. A comparison of the periodontal health of patients during treatment with the Invisalign system and with fixed orthodontic appliances. J Orofac Orthop. 2005 May;66(3):219-29.
5. Demling A, Demling C, Schwestka-Polly R, Stiesch M, Heuer W. Short-term influence of lingual orthodontic therapy on microbial parameters and periodontal status. A preliminary study. Angle Orthod. 2010 May;80(3):480-4. doi: 10.2319/061109-330.1.
6. Van der Veen MH, Attin R, Schwestka-Polly R, Wiechmann D. Caries outcomes after orthodontic treatment with fixed appliances: do lingual brackets make a difference? Eur J Oral Sci. 2010 Jun;118(3):298-303.
7. Deguchi T, Terao F, Aonuma T, Kataoka T, Sugawara Y, Yamashiro T, Takano-Yamamoto T.
Outcome assessment of lingual and labial appliances compared with cephalometric analysis, peer assessment rating, and objective grading system in Angle Class II extraction cases. Angle Orthod. 2015 May;85(3):400-7
8. Grauer D, Proffit WR. Accuracy in tooth positioning with a fully customized lingual orthodontic appliance. Am J Orthod Dentofacial Orthop. 2011 Sep;140(3):433-43.
9. Kravitz ND, Kusnoto B, BeGole E, Obrez A, Agran B. How well does Invisalign work? A prospective clinical study evaluating the efficacy of tooth movement with Invisalign.
Am J Orthod Dentofacial Orthop. 2009 Jan;135(1):27-35
10. Simon M, Keilig L, Schwarze J, Jung BA, Bourauel C. Treatment outcome and efficacy of an aligner technique–regarding incisor torque, premolar derotation and molar distalization. BMC Oral Health. 2014 Jun 11;14:68
11. Liang W, Rong Q, Lin J, Xu B. Torque control of the maxillary incisors in lingual and labial orthodontics: a 3-dimensional finite element analysis. Am J Orthod Dentofacial Orthop. 2009 Mar;135(3):316-22. doi: 10.1016/j.ajodo.2007.03.039.
12. Lombardo L, Stefanoni F, Mollica F, Laura A, Scuzzo G, Siciliani G. Three-dimensional finite-element analysis of a central lower incisor under labial and lingual loads.
Prog Orthod. 2012 Sep;13(2):154-6
13. Dalessandri D, Lazzaroni E, Migliorati M, Piancino MG, Tonni I, Bonetti S. Self-ligating fully customized lingual appliance and chair-time reduction: a typodont study followed by a randomized clinical trial. Eur J Orthod. 2013 Dec;35(6):758-65.
14. De la Cruz A1, Sampson P, Little RM, Artun J, Shapiro PA. Long-term changes in arch form after orthodontic treatment and retention. Am J Orthod Dentofacial Orthop. 1995 May;107(5):518-30.
15. The Damon low-friction bracket: a biologically compatible straight-wire system.
Damon DH. J Clin Orthod. 1998 Nov;32(11):670-80.
16. Aziz, Flores. A systematic review of the association between appliance-induced labial movement of mandibular incisors and gingival recession. Aust Orthod J. 2011 May;27(1):33-9.
17. Cattaneo PM, Treccani M, Carlsson K, Thorgeirsson T, Myrda A, Cevidanes LH, Melsen B. Transversal maxillary dento-alveolar changes in patients treated with active and passive self-ligating brackets: a randomized clinical trial using CBCT-scans and digital models. Orthod Craniofac Res. 2011 Nov;14(4):222-33.
This review looks at links between cardiovascular disease (CVD) and periodontal disease (PD). The literature for the review was acquired by searching databases that were available through UHI multisearch, ResearchGate, Pubmed, Wiley Online Library and the NHS Knowledge Network. The search terms used were cardiovascular disease, heart disease and periodontal disease. From an original 24 papers which were initially found, in total 17 papers fitted the criteria and were analysed for this review. The criteria included: the paper must be a free full text, written in English, from anywhere in the world, must be within 25 years of age, from an unbiased source, subjects could be male and/or female and of any age range.
The links between CVD and PD are often overlooked. In recent years a variety of research and studies have taken place and allowed the links to be strengthened. Despite this there is no mention of the connections on well-known websites such as the British Heart Foundation and the NHS, which are often patients’ first port of call after diagnosis. In this literature review, the target is to provide a detailed analysis of papers to aid the clinician to be able to give a more holistic approach to the treatment provided. 1,2
The link between CVD and PD has been a topic which has been well researched over previous decades and many correlation links have been found, suggesting that the bacteria from the oral cavity enters the blood stream when PD is present and gingival inflammation is existent and thus the bacteria in the blood stream can cause cardiovascular problems by contributing to atheroma formation. 1,2
The effect this has on dental clinicians is that they should have the knowledge to educate and forewarn the patient on the links and increased CVD risks when PD is present. This could help to potentially aid the patient and thus stop the possibility of a fatality. A holistic understanding is the key to first-class patient care. Therefore, the aim of this literature review is to look at how strong the link between CVD and PD is which will then aid clinicians to further their understanding in this area before being competent to pass the understanding on to patients.
Review of papers
The studies that were analysed within this review were no older than 25 years, so as to avoid dated evidence but to keep the selection open for longer studies to be included. They were ranked on the hierarchy of evidence in order to check they were a good source of evidence to be included (Appendices one and two are available in the online version at https://sdmag.wpengine.com).
Only four of the papers looked at were pre-2000 so much of the evidence gathered was within 14 years of this review. With regards to the references, Grau et al. (2004) 4 had a reference 58 years old at time of publication, which is quite old. However, the other references within this review are up to date, and older references are often required to aid in the basic knowledge present today. The papers (DeStefano et al. 1993 5, Joshipura et al. 1996 6, Mattila et al. 1989 7, Mattila et al. 1995 8), which were pre-2000, together had references that were not older than 26 years old at time of publication, meaning they are still relevant and used to date and can aid the reasoning for current research.
The methodology used is similar within the papers, with links between CVD and PD trying to be found by clinical dental examinations and cardiovascular events recorded, although understandably the studies did vary from each other in some areas.
Three of the papers (DeStefano et al. 1993 5, Hujoel et al. 2000 9, Wu et al. 2000 10) look at the same data that was gathered from the First National Health and Nutrition Examination Survey in the US. It was a cohort study that included a dental examination recording number of decayed permanent teeth, oral hygiene index, periodontal classification and periodontal index. Recalls of the subjects happened on four occasions. Each paper looks at the data with slightly different interpretation and includes different sample sizes of different subjects involved, depending on what limitations the authors put in place, meaning there was a difference of between 8,032 and 20,749 included subjects among the studies. Despite this, they were in agreement that there was a correlation. However, they found that the data from this study was inconclusive or statistically inconclusive to PD having a causal association with CVD. Due to the difference in participants numbers, the exact percentages of increased risk varies and because each study presents the data differently, this makes it challenging to compare how different these figures are from each other. Nevertheless, findings which were in agreement were that African-Americans and men are higher risk factors, although this could be due to the fact they are more susceptible to PD and thus the correlation of CVD follows.
A different, yet similar study 11 with a much smaller sample size of 241 coronary heart disease (CHD) patients and 50 control patients, undertook a dental examination making note of the probe depth, recession and bleeding on probing. This study found that CHD patients had significantly higher loss of clinical attachment, probing depth and missing teeth compared to non-CHD patients.
In agreement with the previous papers discussed, 5,9, 10 it also found that men were a common risk factor. In addition, diabetes and smoking patients were found to increase this risk further, which is possibly due to the interconnection between diabetes and PD and how smoking effects the wound healing and thus regeneration, both of these would make the PD more severe. This paper again concluded that there was a positive association between CHD and PD, as in all areas patients with CHD had higher figures (pocket depths, clinical attachment loss, bleeding on probing and missing teeth) than the non-CHD subjects. 12,13
Some studies relied on questionnaires 14, 15, 16 to gather much of their initial data, meaning sample sizes could be larger as costs of clinical examinations are reduced. After the initial information from the questionnaires was gathered, national databases and medical records could be checked to follow cardiovascular events for the remaining study. This allowed the studies to be over a much greater time period and therefore more cardiovascular events can be recorded and the links looked into by the authors.
The Mucci et al. 15 study lasted for 37 years, in which time 15,273 Swedish twins could be considered. It was discovered that the shared genetic factors were responsible for an association between PD and CVD. In addition, twins with severe tooth mobility were at 30 per cent greater risk of developing CVD than those with no tooth mobility. Having mobile teeth means that PD must be present, so this risk can further strengthen the links.
To oppose this, Howell et al. 14 after studying 22,071 US male physicians, concluded that there was no risk factor between CVD and PD. However, it was noted that men who reported PD at baseline had elevated but statistically non-significant increased risk of nonfatal myocardial infarction, stroke and cardiovascular death (10-20 per cent). Although the information is non-significant, it still links in from the study on the increased risk men have found by other studies 5, 9, 10, 11.
Oliveira, Watt and Hamer 16 looked at the links between CVD and oral hygiene. As it is known that it is the bacteria in the plaque, which is the cause of PD, this study can be included. With a sample size of 11,869, it was found that there was no clear difference between age, sex and smokers or non-smokers. In this study it was found that there was a significant association between tooth brushing and CVD, a 70 per cent increased risk if oral hygiene is poor. In addition, frequency of tooth brushing and markers of low-grade systemic inflammation found a direct correlation with C-reactive protein and fibrinogen – the more times teeth were brushed, the lower the number of inflammatory markers. However, it was again concluded that the link between CVD and oral hygiene is present but whether it is a casual link or risk marker is not certain.
When looking at clinical measurements, Machuca et al. 17 undertook a 10-year longitudinal study directly comparing CVD patients and healthy control groups. A dental examination was done at baseline, 12 months and 10 years to try to see if there was any significant difference in the two groups. At baseline, CVD patients did have higher numerical values, but this has been classed as non-significant. After just 12 months, pocket depths were already significantly different with 3.0±1.3 (mm) in the control group and 4.1±1.5 (mm) in the CVD group, but by the 10-year follow-up visit this had once again dropped down to non-significant figures, consistent for clinical attachment loss.
The paper summarised by saying that patients with CVD had bad initial periodontal status and presented a worse response to treatment than the control group. Having said this the CVD patients had a significantly greater plaque index score in all three examinations, so this would effect the treatment. In addition, the control group was selected due to periodontal problems, so this would mean the figures were not accurately based on truly healthy subjects and actually patients who already had deeper periodontal pockets and thus this cannot be classed as genuine health. In addition to this, there were only nine patients in the control group and 35 in the CVD group which is a very small sample size and may not have a wide enough range of ages, sex, socio-economic group etc.
Grau et al. conducted a similar study, although it was a case-controlled study and patients were only examined once. The way it was completed was by examining 303 patients seven days after the cardiac event, including 300 population control patients and 168 hospital control patients. The results showed that the clinical attachment loss mean is higher in the cardiac patient group than both the control groups and this pattern remains for gingivitis, plaque levels and radiological bone loss.
The number of teeth is significantly lower in cardiac patients than the two control groups also. This shows that periodontitis is more severe in this cardiac patient group. The results were all collected in the same way using well-recognised indexes, e.g. Silness and Loe plaque index, allowing the possibility of bias to be reduced. From the results, it shows that an increased severity of periodontitis was associated with an increased risk factor of 4.3 for cerebral ischemia and thus there was a significant association. Looking at Machuca et al. 17 study in comparison it would be good to see Grau et al. 4 study done on a longer term or with follow up visits to see if and how the results change.
The two papers from Mattila et al. 7, 8 looked at the same study although the 1995 paper included an extra series. And, while the 1989 paper does not state dates of the examinations, it is clear they are the same as the 1995 due to sample size of patients, results and that the papers both have fundamentally the same authors. The dentist measured the severity of tooth infection and periodontium after discharge form Helsinki University Central Hospital due to acute myocardial infarction. Patients were then given a score on dental disease of 0-10.
| HIERARCHY OF EVIDENCE |
|---|
| SYSTEMATIC REVIEW/META ANALYSES |
| RANDOMISED CONTROLLED TRIALS WITH DEFINITIVE RESULTS |
| RANDOMISED CONTROLLED TRIALS WITH NON-DEFINITIVE RESULTS |
| COHORT STUDIES |
| CASE-CONTROLLED STUDIES |
| CROSS-SECTIONAL SURVEYS |
| CASE REPORTS |
The 1995 papers results agree with and then go further than the 1989 paper and found that with the additional observations the severity of dental disease correlates with the magnitude of coronary atheromatosis. However, it still only suggests that the association is correlation and not causality. A point that has to be remembered with these studies is that the dentist knew if the subject was a control or patient and so this may cause slightly influenced results.
Joshipura et al. 6 completed a six-year cohort study on 44,119 male health professionals to look at the incidence of coronary heart disease in relation to the number of teeth present and PD. An area, which this study looked at that other studies have overlooked, is the participants diet. Tooth loss can lead to changes in diet and thus increase the coronary heart disease risk. It was discovered that tooth loss was associated with an increased consumption of saturated fats and cholesterol and decreased consumption of fruits, vegetables, carotene and fibre. These food groups are connected with CVD and so this is a possible trigger that has been overlooked in other studies.
At baseline it was discovered that the number of teeth was negatively related to age, smoking and coronary risk factor. There was a significantly higher incidence of coronary heart disease when there were 10 or fewer teeth when compared to full dentition in both groups (positive periodontal history 5.4 per cent to 2 per cent and negative periodontal history 3.74 per cent to 1.42 per cent) with this study having a high follow up rate it thus reduces bias. The overall findings were that no overall association was found between PD and coronary heart disease, although tooth loss may increase the risk, which could mean there was a history of PD.
DeStefano et al. 5 makes a valid point by suggesting that dental infections and coronary artery disease share similar etiological factors, consequently leaving the possibility that it could be poor oral health which is distinctive of an individual’s lifestyle that increases the risk of coronary artery disease, which is a type of CVD. Things such as smoking, diabetes and low socio-economic status are these types of factors. Mattila et al. 7 found that the total dental index correlated significantly with social class, subjects in the lower classes had worse dental health, which agrees with the DeStefano et al. 5 argument.
Within Lamont and Jenkinson’s 18 textbook (p55) the potential causal bacteria are briefly mentioned. However, it is said that evidence is yet to be convinced. With regards to the mechanism of the bacterium on the cardiovascular tissues, papers are very limited – the oldest paper to be looked at is 2009. The studies are all very new with no older papers, as the research itself is still new.
The reviews 19, 20, 21, 22, 23 looked at the types of bacteria, which could cause the links between CVD and PD. Asikainen 20 made the valid point that obtaining valid causal research is difficult due to both conditions being chronic and progressively advancing asymptomatically over many years. In addition, there are vast amounts of data, which require to be interpreted; new methods are being looked at to aid this. It is also said that due to the controversial results of studies, doubts of validity have been raised on the relationship. Despite this, there is still a biologically feasible basis and thus research is continued to take place.
The distinct method of the way in which bacteria causes CVD has been very briefly described within a highly recognised textbook by Clerehugh, Tugnait and Genco 3 (p24-25). However, the paper by Saini, Saini and Saini 23 goes into further detail and describes two theories. Number one being that the oral bacteria affects the heart by attaching itself to fatty plaques within the blood stream and this contributes to clot formation. These clots can block the blood flow and thus lead onto heart attack or other problems.
The second theory is that the PD causes inflammation, which increases the plaque build-up which in turn may contribute to the swelling of arteries. Inaba and Amano 20 agree and comment that due to the clear evidence of the association, control of the oral disease is essential for prevention and management of the condition. According to studies 19, 20 this is because as PD progresses, epithelium develops to be ulcerated. This exposes the connective tissues and blood capillaries, thus allowing bacteria from the plaque biofilm to enter the blood stream. To further this, Kerrigan and Cox 21 gave an explanation saying that once this bacteria has entered the blood stream it forms platelet-bacterial aggregates when contact with platelets is made, these bind to heart valves or are involved with atherosclerosis sites.
Saini, Saini and Saini 23 revealed that there are more than 500 bacterial species, which are capable of colonising within the oral environment. Despite this huge number required to be investigated, P. gingivalis is a bacteria that has come to the attention by having an etiological role in the mechanism. Kerrigan and Cox 21 are in agreement with this but investigated Streptococci ssp. and found that along with P. gingivalis these are the two bacteria that are capable of initiating the platelet activation. In addition, a point was made that several bacteria have evolved so that interactions occur at the same time that get the same response. This makes it more difficult to pinpoint the exact bacteria causing the problem, plus most studies to date have been done under static conditions, which may not reflect the same response as in the fluid environment present within the body.
A final review which gives slightly more information 22 finds that it is the bacteria with strains of serotype-k could circulate through the body more freely and thus there is a possibility that serotype-k S. mutans strains are more likely to be associated with the systemic diseases. Having said this, studies are remaining to take place and it is currently an on-going research process. Due to the research, which has been completed so far being sparse and very new, there is a great need for further research to be completed to strengthen the link and validate the findings further.
Conclusions
The results of the review show that there is a link between CVD and PD, with the majority of studies finding conclusive results of a link, even if this link was small. Howell et al. 14 concluded that there was no risk factor between PD and CVD, despite having slightly elevated figures in the results they gathered. This is due to the fact the results are not significant enough to make this a certainty. This is the case that many of the studies presented with non-significant results, due to their only being a slight difference. This makes it difficult to be confident with the results; however, taking into consideration the age and research done since the uncertainty of these few studies it is clear that a link is present. Older papers were required to look at the progression of knowledge.
This topic is difficult to research due to there being many variables which could influence the results; diet, smoking, diabetes, socio-economic class etc. and thus it is difficult to get an appropriate cohort with all aspects the same to be able to complete a fair assessment of the link. This also means that only a correlation can be made as it may not necessarily be the PD which is the cause of CVD it maybe one of the other factors which is common with PD and CVD which influences the disease.
For a cause to be found further studies have to be done. Within the last five years, studies on the exact bacteria, which could be the cause, have commenced. There are, however, hundreds of bacteria to be researched which is a long task to complete and, therefore, further studies are required to look at all the oral bacteria and how they influence PD and CVD. To date it is P. gingivalis and the serotype-k S. mutans strains which have been found to be the potential cause.
There were a variety of studies analysed in this review. There were a total of five cohort studies that are mid-way on the hierarchy of evidence. Four case-controlled studies were analysed in addition and these fall just below cohort studies. Only one randomised controlled trial was included. However, it was a conclusive study that increases its validity upon the hierarchy further. With regards to the bacterium research, it was mainly reviews which were used – this is due to the fact that there is limited research completed to date on this aspect. Reviews are, however, at the top of the hierarchy so they were good sources from which to extract information. Overall, a good variety of research was used, ideally more randomised, controlled trials would have been included, although they are not available to date.
The papers were difficult to compare due to differences within each study design, e.g. exclusion of different variables, the time period that the study ran for, the number of participants, extent of PD, etc. This could be seen as a positive as it means a greater variety of subjects have been analysed with different study designs at each time which reduces bias.
This review has looked at the potential link between PD and CVD and concluded that the link is present and with studies on the causal bacteria in the initial stages the causal factor is closer to being recognised. Although there are still not enough studies that have been completed to pinpoint the exact bacteria or trigger between PD and CVD. Nonetheless the research process is a long process with this topic in particular as there are so many other conditions within the human body, which need to remain the identical within subjects during investigations.
About the Author
Tegan Dowler graduated from the University of the Highlands and Islands in 2015 as a dental hygienist/therapist. She is currently undertaking her vocational training in Forres within Forres Dental Care.
Further reading
Baratt, H. (2009) The hierarchy of research evidence – from well conducted meta-analysis down to small case series, publication bias. Available from healthknowledge.org.uk/public-health-textbook/research-methods/1a-epidemiology/hierarchy-research-evidence (Accessed 1 November 2014).
British Society of Periodontology. (2014) Periodontal Disease and Treatment. Available from www.bsperio.org.uk/patients (Accessed 18 November 2014).
Hart, C. (1998) Doing a Literature Review: Releasing the Social Science Research Imagination. London: Sage .
NHS choices. (2013) The risks of gum disease. Available from www.nhs.uk/Livewell/dentalhealth/Pages/gum-disease-and-overall-health.aspx (Accessed 18 November 2014).
Peterson, P, E. and Ogawa, H. (2005) ‘Strengthening the Prevention of Periodontal Disease: The WHO Approach’. Journal of periodontology 76 (12), 2187-2193. Available from www.who.int/oral_health/publications (Accessed 18 November 2014).
World Health Organisation. (2013) Cardiovascular diseases (CVDs). Available from www.who.int/mediacentre/factsheets/(Accessed 18 November 2014).
References
1. British Heart Foundation. Cardiovascular disease. Available from bhf.org.uk/heart-health/conditions/cardiovascular-disease.aspx (Accessed 18 November 2014).
2. NHS choices. (2013) Cardiovascular disease – risk factors. Available from nhs.uk/Conditions/cardiovascular-disease/Pages/Risk-factors.aspx
(Accessed 19 November 2014)
3. Clerehugh V, Tugnait A, and Genco R J. (2009) Periodontology at a Glance. Singapore: Wiley-Blackwell
4. Grau A J, Becher H, Ziegler C M, Lichy C, Buggle F, Kaiser C, Lutz R, Bultmann S, Preusch M, and Dorfer C E. (2004) ‘Periodontal Disease as a Risk Factor for Ischemic Stroke’. American Heart Association 35 (2), 496-501 Available from stroke.ahajournals.org/content/35/2/496.long
(Accessed 11 September 2014).
5. DeStefano F, Anda R F, Kahn H S, Williamson D F and Russell C M. (1993) ‘Dental disease and risk of coronary heart disease and mortality’. British Medical Journal 306 (6879), 688-691 Available from ncbi.nlm.nih.gov/pubmed/8471920
(Accessed 20 September 2014).
6. Joshipura K J, Rimm, E B, Douglass C W, Trichopoulos D, Ascherio A and Willett W C. (1996) ‘Poor Oral Health and coronary Heart Disease’. Journal of Dental Research 75 (9), 1631-1636 Available from www.researchgate.net/publication/14254135 (Accessed 1 October 2014).
7. Mattila K J, Nieminen M S, Valtonen V V, Rasi V P, Kesaniemi Y A, Syrjala S L, Jungell P S, Isoluoma M, Hietaniemi K, Jokinen M J and Huttunen J K. (1989) ‘Association between dental health and acute myocardial infarction’. British Medical Journal 298 (6676), 779-781 Available from ncbi.nlm.nih.gov/pmc/articles/PMC1836063/ (Accessed 20 Sept 2014)
8. Mattila K J, Valtonen V V, Nieminen M and Huttunen J K. (1995) ‘Dental Infection and the Risk of New Coronary Events: Prospective Study of Patients with Documented Coronary Artery Disease’ Oxford University Press 20 (3) 588-592 Available from jstor.org.eor.uhi.ac.uk/stable/pdfplus/4458386.pdf (Accessed 20 September 2014).
9. Hujoel P P, Drangsholt M, Spiekerman C and DeRouen T A. (2000) ‘Periodontal Disease and coronary Heart Disease Risk’. The Journal of the American Medical Association 284 (11) 1406-1410 Available from researchgate.net/publication/236292530 (Accessed 20 September 2014).
10. Wu T, Trevisan M, Genco R J, Dorn J P, Falkner K L and Sempos C T. (2000) ‘Periodontal Disease and Risk of Cerebrovascular Disease’. Archives of Internal Medicine 106 (18), 2749-2755 Available from ncbi.nlm.nih.gov/pubmed/11025784
(Accessed 20 September 2014).
11. Rai, B. Kaur, J. Jain, R, K. and Anand, S, C. (2009) ‘Periodontal Disease and Coronary Heart Disease’. JK Science 11 (4), 194-195 Available from web.a.ebscohost.com.eor.uhi.ac.uk/ (Accessed 20 September 2014).
12. Kinane D F and Chestnutt I G. (2000) ‘Smoking and Periodontal Disease’. Critical Reviews in Oral Biology and Medicine 11 (3), 356-365. Available from cro.sagepub.com/content/11/3/356.long (Accessed 20 November 2014).
13. Mealey B L. (2006) ‘Periodontal disease and diabetes’. American Dental Association 137, 26S- 31S. Available from www.ada.org/~/media/ADA/Member%20Center/FIles/Perio_diabetes.ashx
(Accessed 20 November 2014).
14. Howell, T, H. Ridker, P, M. Ajani, U, A. Hennekens, C, H. and Christen, W, G. (2001) ‘Periodontal Disease and Risk of Subsequent Cardiovascular Disease in US. Male Physicians’. Journal of the American College of Cardiology 37 (2) 445-450 Available from ac.els-cdn.com (Accessed 20 September 2014).
15. Mucci L A, Hsieh C, Williams P L, Arora M, Adami H, Faire U, Douglass C W and Pederson N L. (2009) ‘Do Genetic Factors Explain the Association Between Poor Oral Health and Cardiovascular Disease? A Prospective Study Among Swedish Twins’. American Journal of Epidemiology 170 (5), 615-621 Available from aje.oxfordjournals.org/content/170/5/615.full.pdf+html (Accessed 5 October 2014).
16. Oliveira C, Watt R and Hamer M. (2010) ‘Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey’. British Medical Journal 340 (2451) 1-6 Available from www.bmj.com/content/340/bmj.c2451.full.pdf+html (Accessed 20 September 2014).
17. Machuca G, Segura-Egea J J, Jimenez-Beato G, Lacalle J R and Bullon P. (2012) ‘Clinical indicators of periodontal disease in patients with coronary heart disease: A 10 years longitudinal study’. Medicina oral, patologia oral y cirugia bucal 17 (4), 569-574 Available from www.medicinaoral.com/pubmed/medoralv17_i4_p569.pdf (Accessed 20 October 2014).
18. Lamont, R, J and Jenkinson, H, F. (2010) Oral Microbiology at a Glance. Singapore: Wiley-Blackwell
19. Asikainen S E. (2009) ‘Periodontal bacteria and cardiovascular problems’. Future Microbiology 4 (5), 495-498 Available from www.futuremedicine.com/doi/pdf/10.2217/fmb.09.21 (Accessed 20 October 2014).
20. Inaba H and Amano A. (2009) ‘Roles of Oral Bacteria in Cardiovascular Diseases – From Molecular Mechanisms to Clinical Cases: Implication of Periodontal Diseases in Development of Systemic Diseases’. Journal of Pharmacological Sciences 113, 103-109 Available from www.drellie.com/pdfs/Bacteria-in-Cardiovascular-Disease.pdf (Accessed 20 October 2014).
21. Kerrigan S W and Cox D. (2009) The thrombotic potential of oral pathogens. Available from
www.journaloforalmicrobiology.net/index.php/jom/article/view/1999/2342 (Accessed 20 October 2014).
22. Nakano K, Nomura R, Matsumoto M and Ooshima T. (2010) ‘Roles of Oral Bacteria in Cardiovascular Diseases – From Molecular Mechanisms to Clinical Cases: Cell-Surface Structures of Novel Serotype k Streptococcus mutans Strains and Their Correlation to Virulence’. Journal of Pharmacological Sciences 113, 120-125 Available from www.jstage.jst.go.jp/article/jphs/113/2/113_09R24FM/_article (Accessed 20 October 2014).
23. Saini R, Saini S and Saini S R. (2010) ‘Periodontal disease: A risk factor to cardiovascular disease’. Annals of Cardiac Anaesthesia [online] 13 (2), 159-161 Available from annals.in/article.asp?issn=0971-9784 (Accessed 20 October 2014).
The upper airway in humans has multiple purposes: speech, swallowing and breathing. It comprises soft tissue and muscle, but, importantly in the context of this article, does not have hard, bony tissue to prevent the muscle or soft tissue from compressing or collapsing.
The collapsible part of the upper airway, which spans the hard palate to the larynx, can change shape and close briefly to allow for speech and swallowing. It is the collapse of this upper airway during sleep that precipitates an apnoeac event.
Sleep-disordered breathing
The upper airway collapses during sleep when there is no compensatory input to the motor neurones of the upper airway dilator muscles – mostly the genioglossus and tensor palati muscles. How often the person compensates for these collapses determines the rate at which the cycle repeats. When airflow is blocked for 10 seconds or more, it is called apnoea (or apnea) meaning suspension of breathing. Hypopnoea (or hypopnea) is a partial collapse of the airway that results in an airflow reduction of greater than 50 per cent for 10 seconds or more (Figure 1).
Although several neurotransmitters and neuromodulators have been identified as contributing to the regulation of the upper airway opening, there has been little progress finding a medicine to prevent its collapse.
Burwell, Robin, Waley and Bickelmann studied the pathophysiology of sleep apnoea-hypopnoea syndrome (SAHS) in the 1950s. They titled an article published in the American Journal of Medicine in 1956 ‘Extreme obesity associated with alveolar hypoventilation: a Pickwickian syndrome’. This was in homage to Charles Dickens, whose main character Joe in The Pickwick Papers (1836) falls asleep in any situation at any time of day. Pickwickian syndrome can still be found in medical dictionaries to this day.
In 1964, a study showed that 91 per cent of snoring patients had a narrow pharynx, elongated soft palate and uvula. This led in due course to tracheostomy being the first successful treatment – despite serious disadvantages, including recurrent purulent bronchitis and speech difficulties.
Sleep apnoea
Pickwickian syndrome is now commonly called obstructive sleep apnoea (OSA) and we should distinguish between the two types – obstructive sleep apnoea and central sleep apnoea (CSA).
OSA is the result of the mechanical collapse of the upper airway, whereas CSA arises from a reduction or lack of brainstem activity regulating the respiratory muscles activity in failing to send a message to the respiratory muscles to breathe. Each type of apnoea is managed differently.
Where does airway collapse occur?
The upper (pharyngeal) airway from the hard palate to the larynx is made up of hard tissues – the hard palate, the maxilla and mandible, the nasal turbinates, the hyoid bone (anteriorly) and cervical vertebrae (posteriorly) and a collapse of the tube in between.
The pharynx has three segments: from top to bottom the nasopharynx, the oropharynx and the hypopharynx.
The nasopharynx connects the nose to the mouth and remains open when surrounding muscles flex so that the person can continue breathing. The salpingopharyngeal fold and tubal tonsils surround it.
The soft palate (velum or muscular palate) separates the nasopharynx from the oropharynx, which extends from the uvula to the level of the hyoid bone. The oropharynx is divided into the retroglossal pharynx (from the soft palate to the epiglottis) and the hypopharynx (from the epiglottis to the larynx). In addition, the area between the retropalatal and retroglossal pharynx is also called the velum (Fig 2).
OSA and bony structures
We’ll look now at what bony structures can predispose to OSA. Any abnormalities in the hard tissues such as the mandible, maxilla, hard palate and hyoid bone can contribute to the displacement of the soft structures, such as the tongue. This, in turn, can lead to airway obstruction. Bony protuberances along the cervical vertebrae can also lead to airway obstruction.
The most common skeletal abnormally likely to lead to OSA is a short mandible. Shiroh Isono et al published in the Journal of Applied Physiology in April 1997 concluded: “The results support the anatomic hypothesis that sleep apneic subjects have a structurally narrowed and collapsible pharynx.”
Studies have also found that the more inferior the hyoid bone, the more likely the tongue will be displaced lower and potentially increase the risk of developing OSA (Fig 3).
OSA and soft tissues
Typically, someone with OSA will have a disproportionately higher volume of soft tissue compared to their hard tissue cage and any inflammation of these soft tissues may contribute to airway obstruction. Whether increased fat pads also predispose to airway compression is disputed.
Nocturnal rostral distribution, whereby fluid is displaced from the legs to the neck during sleep, can lead to increased pressure in the blood vessels, hence the sort of upper airway collapse seen in patients with, for example, heart failure.
OSA and general factors
Here, I shall discuss how gender, ethnicity, age and obesity can contribute to the pathophysiology of OSA.
OSA is present in twice as many men (around 4 per cent of the population) as women (2 per cent). Incidentally, these figures are only for those diagnosed with OSA – probably only one fifth of all those who have the disease.
It appears men are more likely to experience airway collapse because of their higher pharyngeal resistance, but the effect of testosterone may also be a factor. Women’s risk of having sleep apnoea increases after menopause, and those who have the condition have more severe symptoms than do younger women.
Although there has only been limited research, there are ethnic differences in the prevalence and severity of OSA. For instance, epidemiological studies show African-Americans have a larger tongue and longer soft palate, whereas Asians generally have a shorter maxilla and mandible and lower BMI than Caucasians.
A study by Wen Bun Leong et al published in the Journal of Clinical Sleep Medicine (Vol 09, No. 09) concluded: “OSA prevalence and comorbidities was greater in severely obese South Asians compared to obese white Europeans. South Asians also had more severe OSA compared to BMI-matched white Europeans.”
There is an increased risk of OSA as one gets older. Ageing may lead to reduced muscle tone and hence greater likelihood of airway collapse. NHS Choices states that: “Although OSA can occur at any age, it is more common in people who are over 40.”
By contrast, obesity is a significant factor in the development of OSA. This may be due to the increased fat deposits in the neck resulting in greater extraluminal pressure and airway narrowing. Certainly, it has been shown that fat deposition around the neck is more critical than body mass, which is why neck circumference is such an important measure. NHS Choices states: “Men with a collar size greater than around 43cm (17 inches) have an increased risk of developing OSA.”
Other general factors that may contribute to the pathophysiology of OSA include high blood pressure, diabetes, genetics, smoking, alcohol and drugs (hypnotics and sedatives).
- Figure 1
- Figure 2
- Figure 3
So far, so interesting
Before I move on from the physiology to the pathology, I’ll summarise what we’ve learned so far.
For factors contributing to the pathophysiology of obstructive sleep apnoea, I invariably use the table in an article by Deegan and McNicholas published in European Respiratory Journal in 1995. Incidentally, I recommend this article, Pathophysiology of obstructive sleep apnoea, because it goes into much greater depth than I have room for here. It is available at erj.ersjournals.com
General factors are anthropometric (male sex, age, obesity), drugs (ethanol, hyponotics) and genetics.
Reduced upper airway calibre can be a result of specific anatomical lesions (enlarged tonsils, micrognathia – a lower jaw smaller than normal), neck flexion and nasal obstruction.
Mechanical factors are supine posture, increased upper airways resistance and increased upper airway compliance.
Upper airway muscle function – abnormal upper airways muscle activity; impaired relationship of upper airways muscle and diaphragm contraction.
Upper airway reflexes – impaired response to negative pressure and feedback from the lungs.
Central factors are reduced chemical drives, increased periodicity of central drive and inadequate response to breath loading.
Finally, under the heading of arousal, we have impaired arousal responses and post-apnoeac hyperventilation.
The consequences
Sleep-related breathing disorders (SRBDs) have an adverse effect on the cardiovascular, metabolic, endocrine, nervous and immune systems and the metabolic cycle.
There have been numerous studies on the relationship between SRBDs and cardiovascular consequences. Originally the coexistence of SRBDs with cardiovascular diseases was thought to be due to shared risk factors such as age, gender and obesity. However, Bananian et al published an article ‘Cardiovascular consequences of sleep-related breathing disorders’, in which they stated: “…recent epidemiologic data confirm an independent association between SRBDs and the different manifestations of cardiovascular diseases.” (europepmc.org).
Kathleen A Ferguson and John A Fleetham published an article in Thorax in 1995 which went into great detail about the consequences of sleep disordered breathing (ncbi.nlm.nih.gov).
In relation to cardiac consequences, they cited systemic hypertension, stating: “In patients with sleep disordered breathing, there are brief phasic changes in blood pressure superimposed on a cyclical pattern which coincide with the upper airways obstruction.”
For pulmonary hypertension/right heart failure, they wrote: “The cyclical changes in pulmonary artery pressure parallel the changes in systemic blood pressure.”
They also considered cardiac function – “Treatment of sleep disordered breathing can improve cardiac function in selected patients” – ischaemic heart disease (coronary artery disease) and cardiac arrhythmia (irregular heartbeat).
The Wisconsin Sleep Cohort (WASC) is an ongoing longitudinal study of the causes, consequences and natural history of sleep disorders, particularly sleep apnoea, which has been running for more than 20 years. Research into coronary heart disease as part of WASC, published in SLEEP, the joint publication of the Sleep Research Society and the American Academy of Sleep Medicine, concluded: “Participants with untreated severe sleep disordered breathing (AHI > 30) were 2.6 times more likely to have an incident coronary heart disease or heart failure compared to those without sleep disordered breathing. Our findings support the postulated adverse effects of sleep disordered breathing on coronary heart disease and heart failure.” (see www.journalsleep.org/ViewAbstract.aspx?pid=29996).
AHI stands for apnoea-hypopnoea index – the average apnoeas and hypopnoeas that occur during sleep. Mild OSA is in the AHI scale of five to 15. Moderate OSA is when AHI is between 15 to 30. Severe OSA, with an AHI of 30 or more, could, for example, mean falling asleep while driving.
The metabolic syndrome, including cardiovascular disease, diabetes and stroke, is diagnosed when an individual presents with three or more of the following factors:
- Increased waistline with high BMI
- Increased blood cholesterol HDL
- Increased blood triglycerides
- Increased blood pressure
- Impaired fasting glucose.
Other consequences of SRBDs include cerebrovascular disease, excessive daytime sleepiness, personality and behavioural changes, decreased libido and/or impotence and nocturia (the need to urinate during the night).
For clarity and convenience, I list these consequences of SRBDs below:
- Daytime fatigue with an increased risk of road traffic and work-related accidents
- Decreased cognitive function
- Increased risk of cardiovascular disease
- Increased risk of diabetes
- Eye complications
- Memory loss
- Learning difficulty and growth in children
- Sleep-deprived partners with ensuing marital strife
- Complications during sedation and general anaesthetic for surgery
- Morning headaches
- Mood swings and depression
- Nocturia
- Decreased libido and erectile dysfunction
- Depression
- Insomnia.
Conclusion
Snoring and obstructive sleep apnoea are serious conditions with possible grave consequences, including an increased mortality rate. Dentists can work alongside the medical profession to help with the screening, assessment and subsequent treatment process.
The British Society of Dental Sleep Medicine (BSDSM), of which I am president, is a professional organisation for members of the dental team interested in helping patients seeking help for snoring and obstructive sleep apnoea. It advocates medical diagnosis and the provision of the most appropriate therapy.
The BSDSM runs one-day introduction to dental sleep medicine courses and more information, as well as online booking, is at www.dentalsleepmed.org.uk
About the Author
Dr Aditi Desai works with respiratory, chest and sleep physicians, ENT consultants, neurologists and psychologists helping manage patients with snoring and sleep apnoea at the snoring and sleep apnoea clinic at 76 Harley Street, London. She is also a dental surgeon with special interest in sleep medicine at London Bridge Hospital.
Dr Desai has accreditation from the European Academy of Dental Sleep Medicine and is a member of the American Academy of Dental Sleep Medicine. She is president of the British Society of Dental Sleep Medicine (BSDSM).
The World Health Organisation (WHO) has defined multiple sclerosis (MS) as a “neurological inflammatory demyelinating condition of the central nervous system, that is considered to be autoimmune in nature”.1
In people with MS, the immune trigger is unknown; however, the target is the myelin sheath of the central nervous system tracts. The body attacks the myelin sheath and this forms scar tissue, or sclerosis, which gives the disease its name. When any part of the myelin sheath is damaged, the nerve impulses are distorted or interrupted which will produce a wide variety of symptoms, such as fatigue, tremors, numbness in face and limbs, cognitive dysfunction, and neuropathic pain that can affect a person’s everyday life2.
Multiple sclerosis can have a direct or indirect impact on oral health, be it difficulty cleaning teeth due to reduced muscular power, difficulty accessing dental care due to mobility, or the oral side effects of drugs used for the treatment of MS.
My interest in multiple sclerosis began when my mother was diagnosed with the condition in 2010. I had little knowledge of the disease, and when I began my training as a dental hygiene therapist I was curious to know the effect it has on oral health. As part of my final year of study I decided to carry out an elective project to investigate the oral problems associated with multiple sclerosis and the management of these patients within general dental practices throughout Scotland.
I found that carrying out the project gave me a clearer understanding of the disease, and by sharing my findings through this article, I hope that I can raise awareness of MS and in turn improve the care that these types of patients receive.
A study carried out by the University of Dundee estimated that there are 127,000 people with multiple sclerosis in the UK, and that each year 6,000 people are diagnosed with the condition3. They found that the highest prevalence and incidence rates of MS were observed in Scotland, with 190 cases per 100,0004. Through carrying out a literature review I could see that there is currently a clear lack of studies and research based on oral health and multiple sclerosis. Considering there is such a high population of people diagnosed with MS in the UK, there should be more research into this area.
The National Institute of Clinical Excellence (NICE) recently published updated guidelines for MS management in October 2014. The NICE clinical guideline 186 titled: Multiple Sclerosis: Management of Multiple Sclerosis in primary and secondary care5 has replaced NICE clinical guideline 8 published in November 2003. This updated guideline offers evidence-based advice on the care of adults with MS, and outlines how people with MS can receive better care and improve access to therapies that benefit the condition. This document outlines how the care for patients with MS requires a multidisciplinary approach which includes a variety of health care professionals, but it has not included dental care professionals within this multidisciplinary team.
A number of studies (Fischer et al.6 and Fragoso et al.7) recommend that there should be a dental professional included in the multidisciplinary care team of MS patients.
A paper titled Oral and maxillofacial manifestations of multiple sclerosis by Chemaly et al.8 discussed the three most common oro-facial symptoms presenting in multiple sclerosis: trigeminal neuralgia, trigeminal sensory neuropathy and facial palsy. They suggest that dentists should be aware of the importance of this disease in the diagnosis, treatment and prognosis of oro-facial lesions, and have up-to-date knowledge of the disease.
- Figure 1
- Figure 2
The paper highlights the idea that dental professionals should be prepared to manage a clinically healthy patient that presents with the oro-facial manifestations of MS, and the possibility of referring them for further investigation. They may be involved in the diagnoses of these patients, so it is vital that they have full knowledge of the disease.
Reich and Campbell9 also looked into the various oral implications of MS and how to provide effective care to these patients. They particularly focused on trigeminal neuralgia and how it may be triggered by routine actions such as tooth brushing and mastication. They suggested that patients may be disinclined to continue oral hygiene practices if they trigger painful attacks, so it may be necessary to manage the patient’s systemic disease first, before they can achieve good oral health.
This emphasises the importance of dental professionals being included in an inter-professional care team for MS patients, as dental professionals and neurological specialists will be able to work together to successfully manage the patient’s condition and symptoms, so we can then focus on other aspects like oral health.
Elemek and Almas10 raised the topic of MS and periodontal disease in their paper Multiple sclerosis and oral health: an update. They state that “Multiple sclerosis and periodontal disease both have an inflammatory origin”, and may be linked. However, this requires further investigation. Their paper also discusses that, due to loss of muscular co-ordination and manual dexterity, there is an increased difficulty in maintaining oral hygiene, and as a result, an increased susceptibility to plaque build up which will affect the hard and soft tissues.
They recommend that patients are given specific support such as recommending to use an electric toothbrush. It is also suggested that treatment should be adapted to the individuals needs, for example, longer appointment times, with breaks for relaxation of the facial muscles and frequent urination (which is a common symptom that can occur with MS). These appointments should be carried out in a relaxed environment, and “in the morning, as these tend to be less stressful for patients with neurological problems”10.
The paper also suggests that treatment should be carried out when the patient is in remission. This is when their symptoms can partially or completely subside for a period of time, which can make treatment easier for them and the operator. The clinician should also consider the referring the patient to a hospital outpatient clinic for care. The study also states that people with MS consider their oral health to be important, as they found that a large percentage visited their dentist regularly. I believe that since these people want to have good oral health, the dental care professionals should provide tailored advice and support to these patients so they can maintain their oral health.
Baird11 designed a cross-sectional postal questionnaire- based study to determine the impact that MS has on patient attendance at dental practices, and maintenance of oral health in Leicestershire, UK. This study further supports the idea suggested in the previous paper that people with MS consider their oral health to be important. They found that compared to the general population, a higher proportion of people with MS were registered with a dentist (49 per cent compared with 88 per cent), and they also frequently attended more in the past year (71 per cent compared to 81 per cent). This shows that oral health is very important to these patients, and they may be more aware of their oral health and are conscious to take care of their teeth and mouths.
However, people with MS said that they experienced difficulties attending a dental practice and maintaining their oral health. The greatest problem that was identified which has an impact on attendance was reduced mobility. These patients may face barriers to dental care, particularly as their disability increases.
The study recommends that initiatives are required to increase awareness of the importance of oral health to the quality of life of people with MS, and to ensure that access to dental services for individuals with physical disabilities improves, so that these people receive the same access to care as the able-bodied. It also suggests that patients with a progressive disability may greatly benefit from the provision of preventative oral health care.
A study titled Oral Health Status of a population with Multiple Sclerosis by Santa Eulalia-Trosfontaines et al.12 aimed to determine the oral treatment needs of patients diagnosed with MS in a community in Madrid. They carried out a cross-sectional epidemiological study with a sample of 64 patients aged 25-77 years. They did not specify the type of MS that each individual has.
To evaluate the oral health status, they used guidelines from the WHO. They found that caries prevalence was 100 per cent in all groups, and on analysing gingival health, 65 per cent of people had calculus, 5 per cent had bleeding, and 30 per cent were healthy. The DMFT index found was very similar to that of the general population in that area. However, the gingival health status was poorer in this group of MS patients, and demonstrated that people with MS require specific assistance with their oral hygiene routine, especially in regards to gingival health.
Fischer et al.6 listed the various medications that are prescribed for the management of MS, along with the vast range of side effects that can occur, and in particular the oral side effects, such as: mucositis, ulcerative stomatisis, gingival hyperplasia, xerostomia, postural hypotension, glossitis, and immunosuppression. Drugs taken for MS can treat relapses, modify the disease course or manage the symptoms of the disease13.
- Figure 3
- Figure 4
Disease modifying medications (e.g Copaxone or Tysabri), and steroids for treatment of relapses are the most commonly prescribed medications. However, there are also various medications taken to manage symptoms of the disease such as Amitriptyline, Carbamazepine, and Baclofen. Alternative therapies such as aroma therapy, acupuncture, vitamins and hyperbaric oxygen therapy are popular.
In order to carry out the study for my project, a series of two questionnaires were given to patients with MS (contacted through the MS Central Online Support Group, and Revive MS Therapy Centre), and to general dental practices (to be completed by dentists, dental hygienists or dental hygiene-therapists) throughout Scotland.
I found a strong correlation between the results I had gained with the evidence I discussed in my literature review.
The majority of respondents were diagnosed with Relapsing Remitting MS (Fig 1), and the overall majority stated that they attend a general dental practice for care. The respondents with primary progressive stated that they were treated in a community dental setting, which would be expected, as these facilities offer ease of access and more appropriate specialist treatment suited to the individual patient’s needs. Baird11 supports this as he identified that MS patients face increasing barriers to dental care as their disability increases, and so we need to ensure that access to dental services for these individuals improves.
The patients felt that fatigue, weak hands and arms, poor control and co-ordination all affected their ability to clean their teeth and so can affect oral health. This supports what Reich and Campbell9 found when they looked into the various oral implications of MS and how to provide effective care to these patients. They stated how loss of muscular co-ordination and manual dexterity leads to an increased difficulty in maintaining oral hygiene.
The dental practitioners stated that they advised the use of an electric or large handle tooth brush, adapting the patient’s oral hygiene routine, use of fluoride in all forms, xerostomia advice, and diet advice. The practitioners also stated that when treating a patient they try to ensure the surgery is wheelchair and mobility-aid accessible. They also aim to have lengthy appointment times and to give them lots of breaks. One practitioner stated that they try to keep the appointments to a certain time of day.
From my questionnaires the majority of practitioners stated that they did not feel confident in treating a patient with MS, and felt that they would benefit from guidelines or training. With the clear lack of specific advice and guidelines given it may be hard for practitioners, especially with limited experience, to feel confident in carrying out treatment.
The patient respondents felt that they were not fully aware of the effects the disease can have on oral health.
Both groups of respondents suggested various things that could be done to raise awareness of the oral implications that MS can have – for example, CPD, national guidelines, posters and leaflets.
It is apparent from the results I gained from my questionnaires completed by dentists, dental hygienists and dental hygiene-therapists (Figs 2-4) that some practitioners are aware of the effects that MS has on oral health as well as the effects it may have on receiving dental care. However, with the majority of practitioners stating that they did not feel confident treating a patient with MS, it is clear that there should be more information and training available.
In regards to patients with MS, I can conclude that there should be an increased awareness of the effects that MS can have on oral health, be it the direct effects, such as trigeminal neuralgia, the effects that poor dexterity, facial pain, and fatigue can have on oral hygiene practices, or the oral side effects of medication taken for the management of the disease. Some patients are aware of the effects, but it may greatly benefit the majority patients to have an enhanced awareness.
With the increasing prevalence of multiple sclerosis, especially in Scotland, I believe it is essential that dental care providers have knowledge about the disease, and the effects it has on oral health, so that they can effectively manage the patient in all aspects of dental care.
About the author
Rebecca Wilson qualified as a dental hygiene therapist in 2015, after gaining a Bachelor of Science in Oral Health Science at Glasgow Caledonian University. She currently works as a dental hygiene therapist at Spring Grove Clinic in Auchterarder and Comrie.
References
1. World Health Organization. Atlas: Multiple Sclerosis Resources in the World. 1st ed. Switzerland: World Health Organization; 2008.
2. NHS Choices. Symptoms of multiple sclerosis. 2014. [internet] Available at: nhs.uk/Conditions/Multiple-sclerosis/Pages/Symptoms.aspx
3. Mackenzie I.S, Morant S.V, Bloomfield G.A, MacDonald T.M, O’Riordan J. “Incidence and prevalence of multiple sclerosis in the UK 1990-2010: A descriptive study in the general practice research database”, Journal of Neurology, Neurosurgery, and Psychiatry. 2014. Vol. 85, no. 1, pp. 76.
4. MS Trust. Prevalence and incidence of multiple sclerosis. 2014. [internet] Available at: mstrust.org.uk/atoz/prevalence_incidence.jsp
5. National Institute of Clinical Excellence. Multiple sclerosis: management of multiple sclerosis in primary and secondary care. 2014. (NICE CG 186), [internet]. Available at: nice.org.uk/guidance/cg186.
6. Fischer D, Epstein J, Klasser G. “Multiple sclerosis: An update for oral health care providers”, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2009. Vol. 108, no. 3, pp. 318-327.
7. Fragoso Y, Alves H, Alves L, Alves N, Andrade C, Finkelsztejn A. “Dental care in multiple sclerosis – an overlooked and under-assessed condition”, Multiple Sclerosis. 2009. Vol. 15, no. 9, pp. S263-S263.
8. Chemaly D, Lefrançois A, Pérusse R. “Oral and maxillofacial manifestations of multiple sclerosis”, Journal (Canadian Dental Association. 2000. Vol. 66, no. 11, pp. 600.
9. Reich M, Campbell P. “The oral implications of MS. effectively providing dental care to patients who have multiple sclerosis.” Dimensions of Dental Hygiene. 2010. Vol. 8, no. 1, pp. 52-55.
10. Elemek E. Almas K. “Multiple sclerosis and oral health: An update”, The New York State Dental Journal. 2013. Vol. 79, no. 3, pp. 16.
11. Baird W.O, McGrother C, Abrams K.R, Dugmore C, Jackson R.J. “Verifiable CPD paper: Factors that influence the dental attendance pattern and maintenance of oral health for people with multiple sclerosis”. British Dental Journal. 2007. Vol. 202, no. 1, pp. E4; discussion 40-E4.
12. Santa Eulalia-Troisfontaines E, Martínez-Pérez E, Miegimolle-Herrero M, Planells-Del Pozo P. “Oral health status of a population with multiple sclerosis”, Medicina Oral, Patología Oral y Cirugía Bucal. 2012. Vol. 17, no. 2, pp. e223-E227.
13. Multiple Sclerosis Trust. Drugs and treatments used in the management of MS. 2015. [internet], Available at: mstrust.org.uk/atoz/drugs.jsp.
Pain management
The risk of anti-fungal resistance in dentistry: lessons from a Japanese fungus
Globally since 2009, Candida auris, a fungal species closely related to Candida albicans, has been responsible for a number of… Read more
The anxious patient: Empathy, planning and a team-approach
[ Words: Ilyaas Rehman, BDS (Glas) ] Introduction Introduction This case report documents the treatment provided for a very anxious… Read more
Meaningful milestone
Consultation represents the next step on the journey towards achieving a more effective system of CPD Some dental professionals feel… Read more
Top tips for practicing evidence-based dentistry: Part 3
Introduction Implementing the best available evidence and enabling positive sustainable change in practice is an enviable goal for anyone providing… Read more
The management of a paediatric dental avulsion in general dental practice
Abstract A paediatric dental avulsion is not a clinical scenario that you will be faced with on a daily basis… Read more
Intraoral scanners In dentistry – an update on digital technology
With the introduction of the first intraoral scanner, CEREC (Chairside Economical Restoration of Esthetic Ceramics) by Dentsply Sirona in 1985,… Read more
CBCT and clinical decision-making
Arvind Sharma, BDS(Dund), MSc(Endo), MJDFRCS(Eng), MFDSRCPS(Glas) Arvind Sharma presents the second and final part of a structured critical review to… Read more
Problems of a phobic patient
Day One Ms B attends as a new patient at a local dental surgery complaining of sensitivity in a number… Read more
The power of regeneration
Due to its powerful regenerative properties, platelet rich growth factor Endoret (PRGF) is being increasingly used in many fields of… Read more
Suitability of patients for conscious sedation
Patients suitable to undergo conscious sedation (CS) include those with moderate-severe anxiety, a swallow/gag reflex or a mild learning/physical disability… Read more
Top tips for practicing evidence-based dentistry
In the first article, we explored how to establish a research question using the PICO (Population, Intervention, Comparator and Outcome) method… Read more
Quick, aesthetic restoration
A new patient in her early 30s attended for a check-up. A routine radiograph revealed caries under the amalgam filling… Read more