As a dentist working in general practice it is imperative to ensure that valid consent has been obtained for each individual patient. Following on from the judgement in the Montgomery case in March 2015, which brought the law of consent up to speed with what the GDC’s ethical and professional guidance expected registrants to do, this article looks at the responsibilities of the general dental practitioner. The importance of excellent communication is highlighted in order to provide sufficient and relevant information to the particular patient you have sitting in your dental chair.
The GDC has set out nine principles in their Standards for the Dental Team document. One of the key principles is obtaining valid consent. This document sets out the standards of conduct, performance and ethics that govern you as a dental professional. The guidance applies to all members of the dental team.
Why do we obtain consent?
For a GDP, there are ethical and moral obligations to ensure that your patient understands the treatment proposed and for consent to be valid. Consent is important because any investigation or treatment carried out without a patient’s consent or proper authority may be regarded as assault. This can lead to further investigations resulting in criminal proceedings, an action for damages, a breach in the duty of care and a finding of impaired fitness to practise by the GDC. There are cases where this is exactly what has happened.
Adults with capacity
The test for capacity and the ability of a patient to undertake decisions is set out in the Mental Capacity Act 2005 (MCA) and are supported by the code of practice established under the act, which dental professionals are expected to follow.
In the case of a patient undergoing a dental examination, consent is the expressed or implied permission of a patient to undergo this check-up, investigation and treatment. It is essential that consent is given freely and with adequate understanding of the condition to be treated, the procedures involved, other treatment options and the health implications of giving and withholding consent. It is also important to check that the patient understands the information given.
Adults without capacity
When making decisions on behalf of adults lacking capacity there are a number of points to consider. In Scotland, under the Adults with Incapacity (Scotland) Act 2000, a competent adult can nominate a welfare attorney to make decisions on their behalf should they lose capacity to make those decisions themselves. The law also provides general power to treat a patient who is unable to give consent.
The dental professional responsible for treatment must have completed a certificate of incapacity before any treatment is undertaken, other than in an emergency. Put simply, decide what constitutes a patient’s best interests by taking into account factors other than just their dental condition – treat the patient holistically. Consider consulting with others, including getting a second opinion from a colleague before starting treatment.
Consent and children under the age of 16
Children under the age of 16 can give valid consent to treatment if they are deemed to be Gillick competent. The ability for a child to give valid consent depends on their maturity and understanding. To be Gillick competent, a child must understand the proposed treatment, risks and alternatives, they must be able to retain that information and be able to weigh up the pros and cons of the treatment. The child must be able to communicate that their decision to have the treatment.
If a child is not deemed to be Gillick competent then someone with parental responsibility must provide this authority. It is important to note that emergency care should not be delayed in order to prevent serious harm. In deciding whether or not to treat, the child’s best interests must be considered. Even if a child is Gillick competent, I always encourage an open dialogue between parents and children when it comes to making decisions regarding their health.
Criteria for consent
It is fundamental that valid consent is obtained before starting any treatment. You must make sure that your patient understands the decisions they are being asked to make and that the consent is valid at each stage of the treatment or investigation. Having good communication with your patients is vital in order to obtain valid consent. The way consent is obtained must be tailored to suit the patient’s needs.
As the Standards for the Dental Team states, patients must be given: options for treatment, risks, benefits, why a treatment is necessary and appropriate, consequences and risks of the proposed treatment, prognosis and consequences of not having the treatment, whether the treatment is guaranteed and for how long. Failure to give correct or sufficient information may result in a breach in your duty of care and if proven there was a negligent failure to inform and, as a direct result the patient suffered harm, the patient may take further action.
The cost of any examination, investigation or treatment should also be explained before it starts. It is important to note that a patient who pays the bill has not necessarily consented to treatment. If a patient’s condition changes, causing a change in the proposed risks, then consent must be obtained again, any changes in cost must also be reviewed with the patient. Duress of any form, such as influence from someone else can invalidate consent.
The advice from the Dental Defence Union is to ideally have a ‘cooling off’ period in which the patient can think over their decision and can seek further advice on this if they need to do so. It is best to re-confirm consent with a patient immediately before any treatment. You should also include as much information in your notes about those discussions as possible.
Consent checklist
By developing a logical approach in your daily practice you can ensure that the consent obtained is valid. The patient should be aware of the purpose, nature, and likely effects, risks, chances of success of a proposed procedure, and of any alternatives to it. It is important to note that consent is not open ended and must be obtained again at subsequent occasions. Consent must be obtained for specific procedures, on specific occasions. When the patient is in your dental chair you need to be certain that valid, informed consent has been obtained.
The following checklist is reproduced from the Consent – Scotland publication from Dental Protection Limited (http://bit.ly/DPLconsent)
Ask yourself:
- Is my patient capable of making a decision? Is the decision made voluntary and without coercion in terms of the balance/bias of the information given, or the timing or context of its provision?
- Does my patient need this treatment? Remember, if it is an elective procedure then the onus upon a clinician to communicate information and warnings become much greater. The procedural steps, risks and recovery should be discussed in detail prior to the treatment appointment and the patient should be given adequate time to consider the information given.
- What will happen in the circumstances of this particular case – what will happen if I proceed with the treatment? Is my patient fully aware of my assessment in clear terms? Can I predict the outcome accurately? If not, then what are the areas of doubt and what are the possible alternative outcomes?
- What should a reasonable person expect to be told about proposed treatment? In this case, is there anything specifically important or relevant about my patient? (If in doubt then you are not ready to proceed with the proposed treatment).
- Does information for my patient need to be provided in writing or has my patient requested a wish to have written information? Remember, if you are relying on providing information through marketing material, it is important to make sure that it is presented in a balanced and unbiased manner.
- Accurate and contemporaneous records are imperative. Do my records accurately reflect the conversations with my patient? Will these notes allow me to show what information was given to my patient? On what terms and what was said at what time?
- Does my patient understand what treatment they have agreed to and why? Have they been given the opportunity and adequate time to consider the treatment and its implications, and time to raise concerns and/or have their questions answered?
- Does my patient understand the costs involved? As well as the potential future costs in terms of complications?
- Does my patient need time to consider the treatment options proposed or is my patient wishing to discuss proposals with someone else?
- If I am inexperienced at carrying out the procedure in question, does my patient know that? Is my patient aware that their prospects of a successful outcome if they choose to have the procedure carried out by a specialist or a more experience colleague? Is the technique being used relatively new/untried and does my patient understand this?
References
1. General Dental Council. Standards for the Dental Team. Guidance of obtaining valid consent. GDC; 30 September 2013. Available at https://www.gdc-uk.org/api/files/NEW%20Standards%20for%20the%20Dental%20Team.pdf [Accessed on 21/10/17]
2. Dental Protection. Consent – Scotland. Dental Protection Limited. Available at http://bit.ly/DPLconsent [Accessed on 22/10/17]
3. ISD Scotland. Dental Statistics – NHS Registration and participation. A National Statistics Publication for Scotland; 2017. Available at: www.isdscotland.org/Health-Topics/Dental-Care/Publications/2017-01-24/2017-01-24-Dental-Report.pdf?49161928893 Accessed 30/10/2017
4. Dental Defense Union Guide – Consent guide [Accessed 25/10/17]
5. Harris J, Sidebotham P, Welbury R. Child protection and the dental team. An introduction to safeguarding children in dental practice. Sheffield: Committee of Postgraduate Dental Deans and Directors, 2006. Available at: bda.org/childprotection [Accessed 15/10/2017]
6. Department of Health. National Institute for Clinical Excellence – Consent, procedures for which the benefits and risks are uncertain. Available at https://www.nice.org.uk/guidance/ipg56/documents/consent-procedures-for-which-the-benefits-and-risks-are-uncertain2 [Accessed 30/10/2017]
About the author
Aisha Shafi is a general dental practitioner with a special interest in cosmetic dentistry and facial aesthetics, currently working in clinics based in Glasgow, London and Portsmouth. She was a finalist for Dentist of the Year at the Scottish Dental Awards 2017 and shortlisted for ‘Best Professional’ with the Scottish Asian Business Awards.
Aisha helps run the British Smile Foundation, a group that actively promotes oral health education in the community and is now pending charity registration with the OSCR.
The use of silver-based compounds as antimicrobial agents has been well-documented and common practice for more than 100 years in both medicine and dentistry. From wound dressings to water purification systems, Ag+ is able to destroy pathogens at concentrations of <50ppm. More recently, the use of silver diamine fluoride (SDF) in dentistry has been increasing with applications including caries prevention, arresting carious lesions and the treatment of sensitivity 1.
There are a vast number of products that have been used to deliver fluoride in the aim of preventing caries including milk, salt, toothpaste and varnish. It is thought that where SDF differs is that the silver salt component has a potent antibacterial effect with the ability to encourage the formation of calcified/sclerotic dentine while the fluoride provides a remineralising effect. As such, SDF has stimulated significant interest in the prevention and treatment of caries worldwide based on its ability to reduce instances of pain, ease of use, affordability, non-invasive nature and minimal clinical time for application 1.
SDF is a colourless topical agent with a large number of practical clinical applications in dentistry including 2:
1) Prevention and treatment of high caries risk patients including both children and adults.
2) Prevention and treatment of caries in patients who are medically compromised.
3) Treatment of root surface caries.
4) Treatment of dentine hypersensitivity.
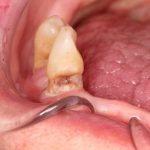
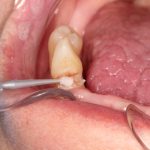
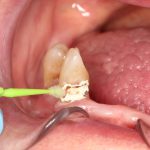
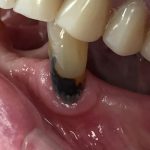
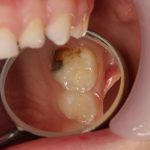
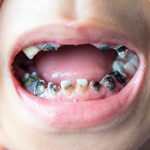
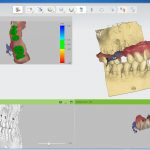
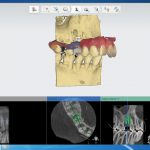
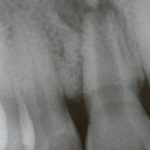
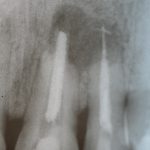
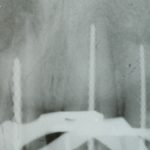
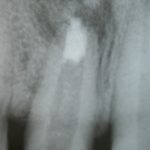
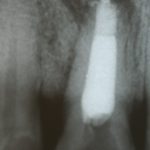
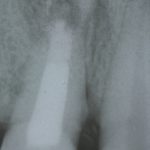
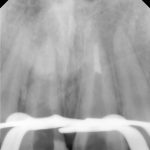
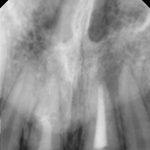
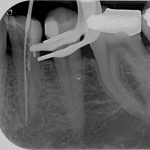
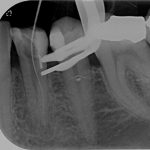
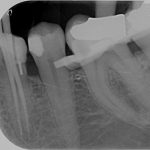
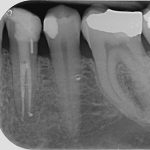
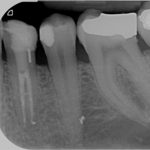
SDF is commercially available in the UK as Riva Star by SDI. The formulation is based on two coloured capsules, silver and green, that must both be applied. Application of SDF is a relatively simple process. The teeth must be cleaned with prophy paste to remove plaque debris before being dried and isolated with cotton wool rolls. If applying close to the gingival margin the kit contains a gingival barrier or alternatively some Vaseline can be placed. The silver capsule is applied first followed by green that causes the formation of a white precipitate. Each capsule can be used to treat around five teeth (see Figs 1-3).
Where being used in the treatment of caries it is important that patients are aware that the aim is to arrest the lesion that will result in a dark appearance (Fig 3). Temporary staining of the gingivae is also possible. Repeated application, twice annually, is essential where the aim is to arrest a carious lesion or to treat dentine hypersensitivity.
- Figure 1. Root surface caries
- Figure 2. Application of Riva Star silver fluoride
- Figure 3. Application of Riva Star potassium iodide
- Figure 4. Post-treatment root surface
- Figure 5. Post-treatment deciduous molar tooth
Caries prevention
A systematic review by Rosenblatt et al. conducted a review of the literature on the use of SDF between 1966 and 2006, identifying 99 papers. The authors were able to conclude that SDF is more effective than fluoride varnish and may be a valuable preventative intervention. They also noted that SDF is a “safe, effective, efficient and equitable caries preventive agent that meets the criteria of the WHO millennium goals”1.
In 2017, a further review of the literature conducted by Contreras et al. found 33 publications meeting the inclusion criteria that were published between 2005 and 2016. The group were able to conclude that SDF is an “effective preventative treatment in a community setting” and that is “shows potential to arrest caries in the primary dentition and permanent first molars”3.
Caries treatment
Chu et al. carried out a study on the use of SDF in arresting carious lesions in 370 Chinese pre-school children aged three to five years old. They compared groups of children receiving SDF treatment, sodium fluoride varnish and a control. The children were followed up for 30 months receiving an intervention every three months.
Children in the SDF groups had a mean of 2.8 arrested lesions compared with a mean of 1.5 in the varnish group. They were able to conclude that the application of an SDF solution was more effective in arresting dentine caries in primary teeth compared with sodium fluoride vanish 4.
Similar results are echoed in a study by Lo et al. which followed 375 Chinese pre-school children over an 18-month period comparing groups of children receiving treatment with SDF, NaF varnish and a control. They found a mean of 0.4 new carious lesions in the SDF treated group compared with 1.2 in the control. They also found similar results in arresting active carious lesions with a mean of 2.8 arrested lesions in the SDF group compared to 1.5 in the NaF varnish group 5.
Clemens et al. treated 118 active lesions with SDF in a community dental clinic in Oregon. They were able to follow up 102 lesions on a three-monthly recall basis and found that 100 lesions were arrested by the first recall and all lesions by the second recall. The authors also noted no incidence of pain or infection and that the parents had a favourable view of the treatment modality 6.
Adults patients have also found a beneficial effect from SDF treatment. Zhang et al. followed up 227 elderly patients over 24 months who were provided with SDF and oral health education compared with a control group. They found a statistically significant result in that the SDF group had fewer root surface lesions than the control group. The authors concluded that SDF combined with oral health education was effective in preventing new root caries and arresting existing lesions in elderly patients 7.
Treatment of sensitivity
Castillo et al carried out a randomised control trial in 126 adult patients experiencing dentine hypersensitivity to assess the effectiveness of SDF as a desensitising agent. They found a reduction in sensitivity at seven days that was statistically significant (p<0.001) compared with the control group and were able to conclude that SDF is a clinically effective desensitising treatment 8.
Guidelines
In October 2017, the American Academy of Paediatric Dentistry issued the first ever evidence-based guideline for the use of SDF in the treatment of dental caries. This followed a systematic review of research between 1969 and 2016. The guideline hopes to lead to a more widespread adoption of SDF as a treatment for dental caries in paediatric and special needs patients 9.
The AAPD describe SDF as the “single greatest innovation in paediatric dental health in the last century aside from water fluoridation” noting the cost effective and pain-free benefits of treatment.
The systematic review on which the guideline is based notes no adverse effects but that a ‘downside’ is the black appearance of cavities. The potential to reduce the number of paediatric cases requiring sedation or GA is high.
The chairside guide suggests that patients who may benefit from SDF include 10:
- High caries risk individuals with active cavitated carious lesions in anterior or posterior teeth
- Patients with additional behavioural or medical challenges who present with cavitated carious lesions
- Multiple cavitated lesions that may not all be treated in a single visit
- Patients with limited access to dental care.
- The chairside guide also suggests the following criteria for tooth selection 9:
- No clinical signs of pulpal inflammation or reports of spontaneous pain
- Cavitated lesions not encroaching on pulp
- Lesions are accessible by a brush to apply SDF (orthodontic separators may be used to help gain access to interproximal regions).
Follow-up is recommended two to four weeks after treatment. Arrested lesions can subsequently be restored. However, where lesions are not restored, biannual re-application is recommended.
In conclusion, silver diamine fluoride is safe and effective in the prevention and treatment of dental caries as well as providing a further treatment modality in the management of dentine hypersensitivity. Application twice annually is a minimally invasive, cost-effective treatment that demonstrates a potentially vast benefit to patients of all ages.
References
1. Rosenblatt A, Stamford T and Niederman R. (2009). Silver Diamine Fluoride: A Caries “Silver-Fluoride Bullet”. Journal of Dental Research, 88(2), pp.116-125.
2. JUCSF protocol for caries arrest using silver diamine fluoride: rationale, indications, and consent. J Calif Dent Assoc. 2016 Jan; 44(1): 16–28.
3. Contreras et al. 2017. Effectiveness of silver diamine fluoride in caries prevention and arrest: a systematic literature review Gen Dent. 2017 May-Jun; 65(3): 22–29..
4. Chu C, Lo E and Lin H. (2002). Effectiveness of Silver Diamine Fluoride and Sodium Fluoride Varnish in Arresting Dentin Caries in Chinese Pre-school Children. Journal of Dental Research, 81(11), pp.767-770.
5. Lo E, Chu C and Lin H. (2001). A Community-based Caries Control Program for Pre-school Children Using Topical Fluorides: 18-month Results. Journal of Dental Research, 80(12), pp.2071-2074.
6. Clemens J, Gold J and Chaffin J. (2017). Effect and acceptance of silver diamine fluoride treatment on dental caries in primary teeth. Journal of Public Health Dentistry.
7. Chu C, Lo E and Lin H. (2002). Effectiveness of Silver Diamine Fluoride and Sodium Fluoride Varnish in Arresting Dentin Caries in Chinese Pre-school Children. Journal of Dental Research, 81(11), pp.767-770.
8. Castillo J, Rivera S, Aparicio T, Lazo R, Aw T, Mancl L and Milgrom P. (2010). The Short-term Effects of Diammine Silver Fluoride on Tooth Sensitivity. Journal of Dental Research, 90(2), pp.203-208.
9. Chairside Guide: Silver Diamine Fluoride in the Management of Dental Caries Lesions. AAPD Reference Manual v 39. No.6, 17/18. Accessed on http://bit.ly/AAPDchairsideguide
About the authors
Michael Dhesi is a GDP who qualified in 2012 with BDS(Hons) from the University of Glasgow and has subsequently completed MFDS RCPS(Glasg) and an MSc in Advanced General Dental Practice at the University of Birmingham. Michael’s focus is in minimally invasive and adhesive restorative dentistry. He also has interests in the management of dental anxiety and oral surgery.
Clive Schmulian qualified from Glasgow University in 1993. Throughout his time in general dental practice, he has developed his clinical skills by obtaining a range of postgraduate qualifications, which in turn led him to develop an interest in digital imaging in both surgical and restorative dentistry. He is a director of Clyde Munro.
We are all too aware that mouth cancer is on the rise. More and more cases are being diagnosed every year with about 300,000 cases of lip and oral cancer reported globally1. In 2014, there were 7,680 cases of oral cancer in the UK2 and, since 1970 there has been a 93 per cent increase in the number of cases3.
Scotland remains a hot spot for oral cancer with higher incidence rates and lifetime risk compared to the rest of the UK.
Cancer Research UK predicts a further 33 per cent increase in oral cancer by 20353. Clearly, we need to act now to address this increasing problem. We, as a profession, have it within our power to do something; stand up, speak out and make some noise about mouth cancer. Our training and position in the community make us the ideal group of health care professionals to provide counsel to patients on risk reduction, screen for the disease and to empower patients with skills and knowledge to find the disease themselves at an early stage.
Risk factors
Traditionally, this has been a disease that affected older men. They have often smoked tobacco and drunk alcohol for many years. Now, that picture is beginning to change. Smoking and alcohol still remain important risk factors but more young people and women are developing this disease without traditional risk factors. Nine out of 10 cases of mouth cancer can be linked to a preventable cause4. Other risk factors include a diet low in fruit and vegetables, poor oral hygiene and the Human Papilloma Virus infection.
With global migration increasing it is likely we will see an increase in the use of smokeless tobacco, areca nut and betel quid in Scotland. There is also growing evidence of the adverse effect that shisha smoking has on health5. Therefore, we must think beyond the traditional risk factors.
A recent study revealed that the vast majority of patients developing head and neck cancer in Scotland are from the most deprived areas in our communities, therefore suggesting that this is a disease of inequality6. In fact, the deprivation gap for mouth cancer is the third highest amongst all cancers at 117 per cent7. Public health initiatives should take this into account when developing measures to address the burden of mouth cancer.
Prognosis
Dentists need to be vigilant when examining and screening our patients; we should have clear protocols and pathways in place for managing suspicious lesions and reviewing those lesions or mouths that simply ‘don’t look right’. Early detection is still recognised as the most important prognostic factor in mouth cancer8. Other prognostic factors include the aggressive nature of the tumour and the proliferation rate of the cells9.
Currently, the mainstay treatment for mouth cancer is high morbidity surgery. As a result of such major surgery, patients’ quality of life post surgery is vastly reduced. Treatment impacts on all aspects of life that we take for granted, such as enjoying meals, conversing freely and showing affection to loved ones. Although there have been great advances using free flap tissue repair to reconstruct surgical excision sites, this has had little to no impact on survival and prognosis, with only 53 per cent of patients surviving to five years post diagnosis10. Those that receive an early diagnosis have an 80-90 per cent chance of survival at five years. While those that present late with advanced disease have a much lower survival or if there is spread to other body systems then treatment is likely to be palliative.
Mouth cancer referral guidance
Dental patients should be examined for signs of malignancy as a part of the routine oral examination at every visit. The Scottish Referral Guidelines for suspected oral cancer11 identify a number of signs and symptoms which may represent malignancy (see Table 1 below). The guidelines recommend that those patients who present with the identified signs and symptoms which last for more than three weeks should be referred urgently to a specialist service according to local referral protocols.
The National Institute for Health and Care Excellence12 (NICE NG12) make similar recommendations and advise that patients who are referred urgently with suspected cancer should be given an appointment in the specialist service within two weeks of referral.
To improve early detection and thus survival, Scottish-based charity Let’s Talk About Mouth Cancer (LTAMC) work directly with the public as well as with professional groups. We advocate a shorter timescale than three weeks, instead recommending that patients should be referred urgently when signs and/or symptoms which are suspicious of mouth cancer do not resolve after just two weeks. The aim of this initiative is to reduce diagnostic delay as much as possible.

Diagnostic delay
Patient delay and professional delay contribute to the total diagnostic delay.
Patient delay
This is defined as “the period between the patient first noticing symptoms and their first consultation with a health care professional concerning those symptoms”13.
Approximately 30 per cent of patients diagnosed with mouth cancer will wait three months following the self discovery of signs and symptoms before attending a doctor or dentist14, 15. This may be because they attribute the symptoms to non-malignant, self-correcting conditions.
A study by Scott et al (2008)13 found that patients with better knowledge of signs and symptoms of mouth cancer are less likely to delay seeking advice. Knowledge about mouth cancer aids interpretation of symptoms and the decision to seek help. The same study also found that a low socio-economic background and deprivation are significantly higher in patients who delay seeking help. These patients also experience real or perceived limited ability to access healthcare. Some of the work of LTAMC aims to rectify some of these issues by educating the public about the signs and symptoms of mouth cancer, focusing on deprived and minority community groups.
Professional delay
It has been shown that lack of knowledge by general dental and medical practitioners about the signs, symptoms and risk factors of mouth cancer can also contribute to the delay in diagnosis. Patients will frequently present first to their doctor with mouth symptoms. A cross-sectional study in Dundee found that, compared to dentists, a significant number of doctors felt they had insufficient knowledge about the detection and prevention of mouth cancer16. Waiting lists and pressures in the health service may also contribute further to professional delay.
How to spot mouth cancer
As recommended in the guidelines, every patient attending for routine check-up should have a full head and neck soft tissue examination. A systematic approach should be routinely used to avoid missing any areas. A video of this can be seen on our website (www.ltamc.org/professional-resources).
Before any examination, a detailed history should be taken. For each area of concern, the patient should be asked about the length of time they have been aware of the lesion/symptoms and ascertain if there has been any pain, change in sensation or effect on function (speech, swallowing, eating). In many cases, however, early and even late tumours can be asymptomatic. It is also worth asking if a lesion has been present before and healed fully or partially. Of course, if the patient is unaware of the area, then questioning may be delayed until after detecting a suspicious lesion.
Extra-orally, the soft tissues should be checked for any asymmetry, swellings or lymphadenopathy; it is important to note any changes in texture and fixation. A hard, fixed lump in the neck is highly suggestive of tumour spread to the lymph nodes.
Intra-orally the oral mucosa has natural variation according to its anatomical site. It is important to be familiar with normal appearances as any changes need to be investigated. The Scottish Cancer Referral Guidelines11 recommend referral for cancer arising from the oral mucosa when there are persistent unexplained lumps, ulceration, unexplained swellings, red or mixed red and white patches of the oral mucosa. Proper and clear description of any lesion is fundamental, both for the sake of good record keeping and also to allow any referral to be as fulsome and informative as possible.
To cover all aspects of a lesion, these characteristics should be recorded and described:
- Site – where the lesion is, note adjacent structures
- Size – can be measured in millimetres with probe/ruler or relative to local anatomy (e.g. extending from mesial 34 to distal 36)
- Colour – red, white or mixed (homogeneous/heterogeneous)
- Texture – hard or soft, fixed or mobile, smooth or rough, induration
- Border – well or poorly defined, raised or flat.
Based on all these findings, a decision must be made whether to monitor in practice, make a routine referral or to refer urgently. It is not necessary to arrive at a definitive diagnosis, rather a decision to refer for further investigation and appropriate treatment. The patient should be informed of the findings, possible diagnosis and also the reasoning for referral or monitoring in practice. The importance of attending arranged appointments must be stressed.
Referrals quiz
Let’s take a few examples and put this into practice. Look at each of the cases below and their brief history. Try describing each as you would for a referral and decide whether you would monitor in practice, make a routine referral or refer urgently. Have a guess at the diagnosis as well. Remember, the triaging surgeon ultimately decides from your referral whether to allocate as urgent or routine, so quality of information is key.
Many lesions are not clear cut and easy to decide on management. You are not alone – if in doubt, seek the opinion of a colleague or send in a referral. In this case, the description and history you submit is essential for the receiving surgeon to adequately assess the urgency for appointment. Be reassured that the majority of urgent referrals after investigation are not cancerous but it is only possible to know that after appropriate tests.
It is vital to follow up a patient where the decision to monitor a lesion within practice or a routine referral has been made. In the instance there are any changes to the area, reconsider if the original decision needs to be altered. Likewise, when managing a lesion in practice first (e.g. ease traumatic denture, smooth sharp edge on tooth, prescribe antifungals), this must be reviewed after two weeks to gauge response. If not healed, then reappraise the suspected cause, treatment provided and potentially send a referral. Also, if the patient misses an appointment or has not received one within the expected time frame, contact the department to ensure one is arranged.
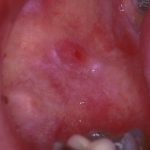
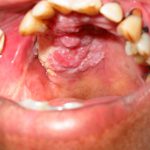
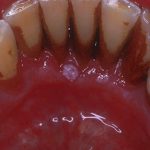
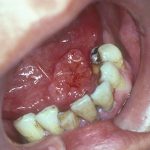
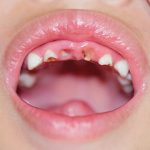
- Case one – Patient: Female, 34, with no symptoms, all feels soft. Clinical description: Stellate reticular white lesion, right buccal mucosa, adjacent to amalgam restoration.
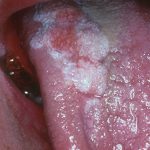
- Case two – Patient: Male, 61, no pain, present for more than three weeks. Smoker. Soft on palpation. Clinical description: Well defined but non-homogenous mixed white and red lesion on dorsum of tongue.
- Case three – Patient: Female, 59, no pain but changed speech for one month. Clinical description: Right lateral tongue raised rolled margins with necrotic centre, firm and fixed on palpation. Hard fixed lumps felt in right neck.
- Case four – Patient: Male, 45, of Asian origin, painful large sore areas for more than three weeks, history of tobacco chewing. Clinical description: Large area of exophytic-type (cauliflower-like growths) affecting maxillary alveolus.
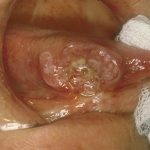
- Case five – Patient: Male, 46, non-painful lesion for one to two weeks. Clinical description: Asymptomatic gingival 3mm in diameter, sessile papillomatous-like lesion.
- Case six – Patient: Female, 55, painful mouth for more than three weeks. Clinical description: Well defined 2cm lesion on floor of mouth with raised rolled margins with necrotic centre, firm and fixed on palpation. Associated left neck swelling.
(Quiz answers are at the end of the article)
Empowering our patients
Recently, LTAMC has developed its strategy away from clinician-based screening in favour of patient empowerment – the focus has changed to teaching self examination for mouth cancer. Although a conventional oral examination by a clinician remains the most sensitive and specific method to detect mouth cancer cases17, teaching mouth cancer self examination empowers patients to recognise pathology in their mouth and may increase awareness.
A Cochrane review published in 2013 found that mouth cancer self examination had similar sensitivity and specificity to breast self examination17. Teaching mouth cancer self examination can be used as a tool in general practice to increase awareness of mouth cancer. It can form part of a general discussion about the signs, symptoms and risk factors of mouth cancer. The thorough, logical mouth self examination process follows five simple steps and is demonstrated in the graphic above. The key messages are to check for: red or white patches; lumps in the mouth that grow; ulcers in the mouth that do not heal; and persistent soreness/discomfort.
The advice is to attend the dentist or general medical practitioner if any of these signs/symptoms do not resolve in two weeks and importantly, to alert the practitioner to a concern about mouth cancer. Patients should be encouraged that mouth self examination is easy, and only requires a light source and a mirror. The aim is simply to empower patients to recognise normal tissues, and to present early if something changes. The mantra “If in doubt, check it out” should be repeated often.
LTAMC has also produced an instructive video aimed at teaching the general public how to perform a mouth cancer self examination. It can be found at youtu.be/WQaujHXauso
The video has been viewed more than 4,500 times in the UK, USA, Japan, Vietnam and India, indicating a worldwide interest. As mentioned above, a recent study has shown that approximately 30 per cent of patients with mouth cancer delay seeking help following discovery of symptoms for more than three months13. As early diagnosis is a key factor for improving prognosis and survival, we as dentists must tackle the lack of recognition of symptoms among our patients.
Lack of insight into initial symptom interpretation and lack of knowledge of mouth cancer have been shown to be significant variables which contribute to patient delay in seeking help13, and are issues that may be easily modified with targeted interventions by general dental practitioners.
Conclusions
Mouth cancer is increasing at an alarming rate and yet large sections of the public know little of the risk factors or signs and symptoms of the disease. Despite the fact that about half the population attend a dentist regularly for dental check-ups, many cases present at a late stage with a correspondingly poor prognosis. Survival has not improved substantially in the past 50 years and there is no treatment available that can transform the poor survival of someone with late stage disease to the much better survival of someone with early stage disease; the only thing that can do this is early diagnosis.
The work of LTAMC is firmly focused on teaching the public as well as professionals to diagnose mouth cancer early. We hope to break down health inequalities and empower as many people as possible to recognise the disease and its risk factors to improve survival. We call it our empowerment journey, so “Let’s talk about mouth cancer!”
Quiz Answers:
Case one
Outcome: Monitor in practice.
Diagnosis: Lichenoid lesion/lichen planus.
Case two
Outcome: Urgent referral.
Diagnosis: Dysplastic lesion or early cancer.
Case three
Outcome: Urgent referral.
Diagnosis: Squamous cell carcinoma.
Case four
Outcome: Urgent referral.
Diagnosis: Squamous cell carcinoma.
Case five
Outcome: Routine referral.
Diagnosis: Human Papillomavirus related papilloma.
Case six
Outcome: Urgent referral.
Diagnosis: Squamous cell carcinoma.
About the authors
This article was co-authored by the five trustees of the Scottish charity Let’s talk about mouth cancer (SC045100):
- Dr Niall McGoldrick BDS MFDS RCPS(Glasg), specialty registrar in dental public health.
- Mr Ewan MacKessack-Leitch BDS(Hons) MFDS RCPS(Glasg), GDP, Fife.
- Dr Stephanie Sammut FDS (OS), MClinDent, MOral Surg, MFDSRCPS,
consultant oral surgeon. - Dr Orna Ni Choileain BDS(Hons) MFDS RCSEd, medical student,
Trinity College Dublin. - Prof Victor Lopes PhD FRCS, consultant maxillofacial surgeon.
Website: www.ltamc.org
Twitter: @couldbeUrmouth
Facebook: www.facebook.com/letstalkaboutmouthcancer
References
1. N Johnson, A Chaturvedi: Global burden of oral cavity and pharyngeal cancers, Global Oral Cancer Forum: 2016
2. C R Smittenaar, K A Petersen, K Stewart, N Moitt. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016 Oct 25; 115(9): 1147–1155
3. www.cancerresearchuk.org [Internet]. 2017 Available from:
www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oral-cancer#heading-Zero
4. www.cancerresearchuk.org [Internet]. 2017 Available from:
www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers/risk-factors#heading-Zero
5. Ziad M El-Zaatari, Hassan A Chami, Ghazi S Zaatari Health effects associated with waterpipe smoking. Tob Control. 2015 Mar; 24(Suppl 1): i31–i43.
6. Purkayastha M, McMahon AD2, Gibson J, Conway DI. Trends of oral cavity, oropharyngeal and laryngeal cancer incidence in Scotland (1975-2012) – A socioeconomic perspective. Oral Oncol. 2016 Oct; 70-5. doi: 10.1016/j.oraloncology.2016.08.015. Epub 2016 Aug 31.
7. www.cancerresearchuk.org [Internet]. 2017 Available from
www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/deprivation-gradient#heading-One
8. Gómez, I., Seoane, J., Varela-Centelles, P., Diz, P. and Takkouche, B. (2009), Is diagnostic delay related to advanced-stage oral cancer? A meta-analysis. European Journal of Oral Sciences, 117: 541–546. doi:10.1111/j.1600-0722.2009.00672.x
9. Warnakulasuriya S. Prognostic and predictive markers for oral squamous cell carcinoma: The importance of clinical, pathological and molecular markers. Saudi J Med Med Sci 2014;2:12-6
10: www.cancerresearchuk.org [Internet ]Available from
www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers/survival#heading-Zero
11. Healthcare improvement Scotland. Scottish referral guidelines for suspected cancer 2013 ( updated in August 2014) available online
www.healthcareimprovementscotland.org/our_work/cancer_care_improvement/programme_resources/scottish_referral_guidelines.aspx [last accessed 31 July 2017]
12. National Institute for health care and excellence. Suspected cancer: recognition and referral – NICE Guideline NG12. 2015 (last updated July 2017) available online www.nice.org.uk/guidance/ng12/resources/suspected-cancer-recognition-and-referral-pdf-1837268071621 [last accessed 31 July 2017]
13. Scott S, McGurk M, Grunfeld E. Patient delay for potentially malignant oral symptoms. Eur J Oral Sci. 2008;116 2:141–147.
14. Scott SE, Grunfeld EA, McGurk M. Patient’s delay in oral cancer: a systematic review. Community Dent Oral Epidemiol. 2006;34 5:337–343.
15. Allison P, Locker D, Feine JS. The role of diagnostic delays in the prognosis of oral cancer: a review of the literature. Oral Oncol. 1998;34 3:161–170. [PubMed]
16. Carter L, Ogden GR Oral Cancer awareness of general medical and general dental practitioners Br Dent J. 2007 Sep 8;203(5):E10; discussion 248-9. Epub 2007 13 Jul.
17. Walsh T, Liu JLY, Brocklehurst P, Glenny AM, Lingen M, Kerr AR, Ogden G, Warnakulasuriya S, Scully C. Clinical assessment to screen for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database of Systematic Reviews 2013, Issue 11.
I’ve been teaching in the area of child protection and dentistry for approximately eight years and completed a masters by research in 2013 on Oral Disease in Vulnerable Children and the Dentist’s Role in Child Protection1. I’m now doing a PhD looking at what is involved in the decision by dental team members to refer suspected cases and how serious game methodology might support this context.
What is clear to me is that often decisions in this area are difficult and uncomfortable to make, so this article looks at what is expected of us as members of the dental team (whether we are dentists, dental nurses, therapists or technicians) and what this means from a practical point of view in our daily working lives.
The General Dental Council states that all members of the dental team “must raise any concerns you may have about the possible abuse or neglect of children” and “must know who to contact for further advice and how to refer concerns to an appropriate authority”2. They also state “you must find out about local procedures for the protection of children” and “you must follow these procedures if you suspect a child or vulnerable adult might be at risk because of abuse or neglect”2.
These wide-ranging statements mainly cover child protection (defined as activities undertaken to protect specific children who are suffering, or are at risk of suffering significant harm) but also bring in elements of safeguarding (defined as measures taken to minimise the risk of harm to all children).
From a practical point of view, there is the need to identify what your concerns are. This could be anything from unexplained (or inadequately explained) injuries, concerns about dental neglect, concerns about general neglect, a general lack of engagement with dental services to witnessing a child being physically abused in your waiting room or surgery. It is the vast spectrum of these concerns which make it difficult to provide what so many people ask for – a step-by-step guide for any conceivable situation – because there are so many different situations that could present themselves.
Some health boards have produced flowcharts for dental teams to follow and there is also a summary flowchart available on the Child Protection and the Dental Team website which are very helpful3. For some of these situations, the dental team members that I have been privileged to speak to (during my research and teaching) find the decision of what to do next straightforward, but for other situations it is more difficult.
It has already been well documented that there remains a 26 per cent gap between the proportion of general dental practitioners who have suspected child abuse or neglect in one or more of their paediatric patients (37 per cent) and the proportion that have referred suspected cases (11 per cent)4. Quantitative methods have consistently shown that the gap between dentists who suspect and refer in Scotland is affected by lack of certainty of the diagnosis, fear of violence to the child, fear of consequences to the child from statutory agencies, lack of knowledge of referral procedures, fear of litigation, fear of violence to the general dental practitioner and concerns of impact on dental practices4, 5.
So, let’s explore four of the most common things I’m asked about and hopefully it will be helpful to all of the team.
1. The child with caries whose family don’t engage with services
I’m asked about this situation a lot, probably because it is a common occurrence. We know caries is still common in children and statistics say 94 per cent of children in Scotland are registered with a general dental practitioner, with 85 per cent having seen their dentist in at least the last two years6. Perhaps a dentist has referred a patient for extractions under general anaesthetic but the patient is never taken to the assessment appointment and the dentist gets a letter back from the public dental service or the hospital dental service discharging them back to their care because they’ve not managed to see them.
Or perhaps it is a family that come for their check-ups but don’t bring the children back to have their treatment completed, or ones who repeatedly cancel, or don’t book check-ups when they get their reminder letters and the dental teams only end up seeing them sporadically. Or it may be children who have required extractions under general anaesthesia for removal of all their primary teeth but then don’t come back until they are aged seven or so and now have unrestorable caries in all their permanent molars.
These situations are difficult and all of them are examples of dental neglect, which is defined by the British Society of Paediatric Dentistry as “the persistent failure to meet a child’s basic oral health needs, likely to result in the serious impairment of a child’s oral or general health or development”7. Although dental caries is still common in children, signs such as failing to complete courses of dental treatment, failing to listen and act on preventive advice given by dental teams, children returning in pain repeatedly, children requiring repeated general anaesthetics due to dental issues or children who are repeatedly not brought to their dental appointments, are all concerning patterns of behaviour which are likely to result in the impairment of a child’s oral or general health or development7,8.
Untreated dental caries may be one of the first signs of child abuse or neglect9. Neglect should be considered if parents have access to, but persistently fail to obtain treatment for their child’s tooth decay10. Research also suggests that abused/neglected children are more likely to have untreated decayed teeth, significantly more dental plaque and gingival inflammation than non-abused/non-neglected children11–13.
Many practitioners whom I have spoken to then say: “Well if I have to refer every patient like that, I would be referring at least 60 per cent or more of my paediatric cohort!” This really depends on what practitioners mean by the term ‘refer’. All of these situations do require some action to be taken, but not all will necessitate an immediate referral to social work. There is very sensible advice given for this type of scenario in Child Protection and the Dental Team (CPDT) which recommends a three-level response to concerns about dental neglect, namely preventive dental team management, preventive multi-agency management, child protection referral3.
Preventive dental team management involves “raising concerns with parents, offering support, setting targets, keeping records and monitoring progress. The initial focus should be on relief of pain accompanied by preventive care. In order to overcome problems of poor attendance, dental treatment planning should be realistic and achievable and negotiated with the family”3. This is often all that is required and, in reality, is probably what most dental teams do on a day-to-day basis (although there is no evidence from research to prove this is the case).
Fully implementing a preventive dental team management strategy can have impacts on a practice and those impacts will have to be discussed as a team, for example deciding who will deal with contacting the family if agreed appointments are cancelled or missed. The CPDT website gives an example of how it might be put into practice3. The areas where research has suggested dental teams could improve upon are ‘setting targets’ and ‘monitoring progress’14. If this level of response to the concerns is not working or there is a breakdown of communication or the child/family has more complex needs then preventive multi-agency management may be more appropriate.
Preventive multi-agency management involves liaising with other professionals who might be involved with the family. Examples of other professionals could include the health visitor (for pre-school children), the general medical practitioner, the child’s social worker (if they already have one) or the child’s named person. The aim of this liaison is “to see if concerns are shared and to clarify what further steps are needed”3. There is a sample letter to a health visitor freely available on the CPDT website which can be used to assist with multi-agency working for children under five years old3.
A joint plan of action should be agreed (for example as a dental practice we will arrange appointments on these dates and the child’s social worker will facilitate attendance, or the health visitor will arrange a home visit by a dental health support worker to facilitate registration and attendance at our dental practice). A date should be specified for review of the action plan so that it can be checked that progress is being made.
If there is any point in the processes above where things begin to deteriorate, or if it is felt at any time that the child is at risk of suffering significant harm (this can include things like a child being in pain for more than a couple of days due to toothache and this is happening on more than one occasion), then any member of the dental team can make a child protection referral. Some dental team members struggle to work out when things become significant. A good rule of thumb can be that if you wouldn’t let children in your own circle of family or friends go through it, then it is probably significant.
Child protection referrals should be made according to the local procedures of where you work. In Scotland, a child protection referral is made either to the police or local children’s social work team (referrals can also be made to the Children’s Reporter but follow your local guidelines). If you do not already know your local contact numbers you can, currently, find them out by visiting www.withscotland.org/public-local-councils and typing in the postcode of the child you are concerned about. (Please note: This website address is likely to change in the future as WithScotland no longer exists, but many of their functions have been taken over by the new Centre for Child Wellbeing and Protection www.stir.ac.uk/ccwp/)
- Caries in children can be evidence of neglect at home
2. How do I make a child protection referral?
The majority of child protection referrals will involve a telephone call to your local social work office (Children and Families office ideally but in many areas in Scotland you will go through Social Care Direct) in the first instance explaining your concerns and stating you wish to make a child protection referral. Write down the names and job titles of everyone you speak to. The telephone referral should then be followed up in writing normally within 48 hours. This may involve completion of a shared referral form, or notification of concerns form (same form just different names), or similar, with one copy going into the child’s dental notes, one copy sent to the social work office that you spoke to on the phone, and, depending on your local procedures, another copy may be sent to your local child protection unit (CPU) or similar (or you may just have to notify your CPU by email or phone).
3. Will the family know I’ve referred them?
The short answer to this is that they might. In most situations it is best practice to tell the family what your concerns are and why you are referring them to social services but there will, of course, be some situations where the family don’t know, either because you can’t get in contact with them or you may believe that you would put the child in more danger if the family were aware of the referral.
You can refer anonymously but, bear in mind that if the concerns you have are related to non-attendance with you or concerns about something dental, then even if you refer anonymously the family will, probably, be able to work out where the referral came from so it is a much better situation if you have informed them the referral is being made.
4. I’m worried about how the family will react
Many dental professionals assume telling a family you are going to contact social work will be bad news, but for some families it will be the first time anyone has actually offered them any help. As members of the dental team, we quite often have to break bad news to our patients (e.g. “I’m sorry I can’t save the tooth, it needs to be extracted”). Being concerned is a natural human response but it is helpful to think through all the reactions that you would be worried about and how, as a team, your practice will manage them.
For example, if the family are angry and choose to de-register from your practice, you can’t always prevent that from happening but you would want to pass that information on to the other agencies involved such as the social work office you referred to. I suggest being quite clear in your practice about what your professional responsibilities are and having posters or information up in waiting areas promoting that you take the safeguarding of children and vulnerable adults seriously.
Many dental team members have told me they are worried that as they live in a small town that word will spread, or their own children will be targeted at school or they will be threatened by the families involved. My advice is that if you are threatened, inform the police and relevant social work office involved. If your own children get picked on because of rumours, approach the school as you would do about any episode of bullying your child may experience and talk to your child about the nature of your job (e.g. “You know mummy/daddy is a dentist/dental nurse/practice manager and looks after people’s teeth, but I also have a responsibility to make sure the children that I see at work are alright and are being looked after properly”). If your practice gets branded as “the ones who call social work”, take this as a good thing as it means you are actively looking out for and promoting the welfare of your paediatric patients. There are many experts out there who can give advice on how to use it as a
‘practice builder’.
Conclusion
Ultimately, it is not only our professional responsibility, but also an ethical responsibility to protect and safeguard those in society who can’t do it for themselves. Doing nothing when you have a concern is never an option – you would probably continue to worry and you cannot predict what the impact on the child would be.
Unfortunately, I have had to look at statements from dental team members when something awful has happened to one of their paediatric patients, and so often there have been warning signs (e.g. multiple missed appointments, failure to complete treatment) but the dental teams did not record or raise any concerns. Clearly I have the benefit of hindsight and experience but my hope is that as more dental teams think about and practise looking out for the wellbeing of their paediatric patients, then perhaps I’ll see fewer awful things happening. Or, if they are still happening, I’ll see real evidence that the dental teams involved did everything they could to help the child.
About the author
Christine Park is a senior clinical university teacher at Glasgow Dental Hospital and School, and honorary consultant in paediatric dentistry at NHS Greater Glasgow and Clyde.

References
1. Harris CM. Oral disease in vulnerable children and the dentist’s role in child protection [MSc Thesis] Glasgow: University of Glasgow; 2013. Available at: http://theses.gla.ac.uk/4150/1/2013harrismsc.pdf.pdf Accessed 03/08/2017
2. General Dental Council. Standards for the Dental Team. Guidance on Child Protection and Vulnerable Adults. GDC; 2013. Available at www.gdc-uk.org/professionals/standards/team Accessed 03/08/2017
3. Harris J, Sidebotham P, Welbury R et al. Child protection and the dental team. An introduction to safeguarding children in dental practice. Sheffield: Committee of Postgraduate Dental Deans and Directors, 2006. Available at: bda.org/childprotection Accessed 03/08/2017
4. Harris CM, Welbury, R, Cairns, AM. The Scottish dental practitioner’s role in managing child abuse and neglect. Br Dent J 2013; 214(E24):1–5. Available at: dx.doi.org/10.1038/sj.bdj.2013.435 Accessed 03/08/2017
5. Cairns AM, Mok JYQ, Welbury RR. The dental practitioner and child protection in Scotland. Br Dent J 2005; 199(8):517–520; discussion 512; quiz 530–531.
6. ISD Scotland. Dental Statistics – NHS Registration and Participation. A National Statistics Publication for Scotland; 2017. Available at:
www.isdscotland.org/Health-Topics/Dental-Care/Publications/2017-01-24/2017-01-24-Dental-Report.pdf?49161928893 Accessed 03/08/2017
7. Harris JC, Balmer RC, Sidebotham PD. British Society of Paediatric Dentistry: a policy document on dental neglect in children. Int J Paediatr Dent 2009. Available at: http://bspd.co.uk/Portals/0/Public/Files/PolicyStatements/Dental%20Neglect%20In%20Children.pdf Accessed 03/08/2017
8. Balmer R, Gibson E, Harris J. Understanding child neglect. Current perspectives in dentistry. Prim Dent Care 2010; 17: 105–109.
9. Blumberg ML, Kunken FR. The dentist’s involvement with child abuse.
NY State Dent J 1981; 47:65–69.
10. National Collaborating Centre for Women’s and Children’s Health (2009). When to suspect child maltreatment: full guidance. Clinical Guideline 89. National Institute for Health and Clinical Excellence. Royal College of Obstetricians and Gynaecologists: London. Available at www.nice.org.uk/guidance/cg89/evidence/cg89-when-to-suspect-child-maltreatment-full-guideline2 Accessed 03/08/2017
11. Greene PE, Chisick MC, Aaron GR. A comparison of oral health status and need for dental care between abused/neglected children and non-abused/non-neglected children. Pediatr Dent 1994;16:41-45
12. Valencia-Rojas N, Lawrence HP, Goodman D. Prevalence of early childhood caries in a population of children with history of maltreatment. J Public Health Dent 2008;68(2):94-101
13. Montecchi PP, Di Trani M, Sarzi Amadè D, et al. The dentist’s role in recognizing childhood abuses: study on the dental health of children victims of abuse and witnesses to violence. Eur J Paediatr Dent 2009; 10(4):185-187.
14. Harris JC, Elcock C, Sidebotham PD, Welbury RR. Safeguarding children in dentistry: 2. Do paediatric dentists neglect child dental neglect? Br Dent J 2009;206: 465 – 470
In 2011, the Scottish Dental Clinical Effectiveness Programme (SDCEP) published clinical guidance on the Oral Health Management of Patients Prescribed Bisphosphonates. This was in response to reports in the literature describing a rare side effect in patients treated with these drugs, bisphosphonate-related osteonecrosis of the jaw (BRONJ)1.
Bisphosphonates are prescribed to reduce bone resorption in patients with osteoporosis and other non-malignant diseases of bone and to reduce the symptoms and complications of metastatic bone cancer. The drugs persist in the body for a significant period of time; alendronate has a half-life in bone of around 10 years2. As dental extractions appeared to be a risk factor for this oral complication, there was a need for guidance providing clear and practical advice for dentists in primary care on how to provide care for patients prescribed these drugs.
Since 2011, several other drugs have been implicated in what is now referred to as medication-related osteonecrosis of the jaw (MRONJ). The condition has been observed in patients treated with the anti-resorptive drug denosumab which, like the bisphosphonates, is indicated for the prophylaxis and treatment of osteoporosis and to reduce skeletal-related events associated with metastasis. Another drug class implicated in MRONJ is the anti-angiogenics, which target the process by which new blood vessels are formed and are used in cancer treatment to restrict tumour vascularisation. At the time of writing, the Medicines and Healthcare products Regulatory Agency (MHRA) has published Drug Safety Updates warning of the risk of MRONJ for three anti-angiogenic drugs: bevacizumab, sunitinib and aflibercept3,4.
Development of the updated SDCEP guidance
In response to these developments, SDCEP convened a second guidance development group (GDG) in 2015 to update the guidance. The GDG included consultants of various dental specialities, primary care dental practitioners, medical specialists and two patient representatives, who provided feedback on patient views and perspectives.
Pre-publication research was carried out by TRiaDS (Translation Research in a Dental Setting, www.triads.org.uk), who work in partnership with SDCEP, including a national survey of users of the first edition of the guidance and interviews with dentists, pharmacists and doctors. The findings informed the updating of the guidance and have been used as the basis for an implementation questionnaire and a national research audit.
A systematic and comprehensive search of the literature was conducted to inform the recommendations in the guidance. The quality of the evidence and strength of each of the key recommendations is stated clearly in the guidance with a brief justification in the accompanying text. A more in-depth explanation of the evidence appraisal and formulation of recommendations is provided in an accompanying methodology document.
NICE has accredited the process used by SDCEP to produce Oral Health Management of Patients at Risk of Medication-related Osteonecrosis of the Jaw, which means users can have high confidence in the quality of the information provided in the guidance. Accreditation is valid for five years from 15 March 2016. More information on accreditation can be viewed at www.nice.org.uk/accreditation
Prior to publication, the guidance was scrutinised through external consultation and peer review and it is endorsed by several of the Royal Colleges, Public Health England and Department of Health (Northern Ireland).
Medication-related osteonecrosis of the jaw
MRONJ was first reported by Marx in 20031 and is defined as exposed bone, or bone that can be probed through an intraoral or extraoral fistula, in the maxillofacial region that has persisted for more than eight weeks in patients with a history of treatment with anti-resorptive or anti-angiogenic drugs, and where there has been no history of radiation therapy to the jaw or no obvious metastatic disease to the jaws5. Risk factors include the underlying medical condition for which the patient is being treated, cumulative drug dose, concurrent treatment with systemic glucocorticoids, dentoalveolar surgery and mucosal trauma. It is important to note that MRONJ is a rare side-effect of treatment with anti-resorptive and anti-angiogenic drugs and, although invasive dental treatment is a risk factor, it does not cause the disease.
At present, the pathophysiology of the disease has not been fully determined and current hypotheses for the causes of necrosis include suppression of bone turnover, inhibition of angiogenesis, toxic effects on soft tissue, inflammation or infection5. It is likely that the cause of the disease is multi-factorial, with both genetic and immunological elements.
Incidence
Estimates of incidence and prevalence vary due to the rare nature of MRONJ. It appears clear that patients treated with anti-resorptive or anti-angiogenic drugs for the management of cancer have a higher MRONJ risk than those being treated for osteoporosis or other non-malignant diseases of bone. This is likely to be due, in part, to the substantially larger doses of the drugs that cancer patients receive.
For patients being treated with anti-resorptive or anti-angiogenic drugs for the management of cancer, the risk of MRONJ approximates 1 per cent5–9, which suggests that each patient has a one in 100 chance of developing the disease. However, the risk appears to vary based on cancer type and incidence in patients with prostate cancer or multiple myeloma may be higher.
For patients taking anti-resorptive drugs for the prevention or management of non-malignant diseases of bone (e.g. osteoporosis, Paget’s disease), the risk of MRONJ approximates 0.1 per cent or less2,5,7, 10–17, which suggests that each patient has between a one in 1,000 and one in 10,000 chance of developing the disease (Table one).
Patients who take concurrent glucocorticoid medication or those who are prescribed both anti-resorptive and anti-angiogenic drugs to manage their medical condition may be at higher risk.
The incidence of MRONJ after tooth extraction is estimated to be 2.9 per cent in patients with cancer and 0.15 per cent in patients being treated for osteoporosis18.

Risk factors
As outlined previously, the most significant risk factor for MRONJ is the underlying medical condition for which the patient is being treated. Dentoalveolar surgery, or any other procedure that impacts on bone, is also a risk factor, with tooth extraction a common precipitating event19–22. However, MRONJ can occur spontaneously without the patient having undergone any recent invasive dental treatment.
The MRONJ risk in patients who are being treated with bisphosphonates is thought to increase as the cumulative dose of these drugs increases. One study found a higher prevalence of MRONJ in osteoporosis patients who had taken oral bisphosphonates for more than four years compared to those who had taken the drugs for less than four years13. There is currently no evidence to inform an assessment of MRONJ risk once a patient stops taking a bisphosphonate drug. Therefore, it is advised that patients who have taken bisphosphonate drugs in the past should continue to be allocated to the risk group they were assigned to at the time the drug treatment was stopped.
The effect of denosumab on bone turnover diminishes within nine months of treatment completion14, 23. Therefore, patients who have stopped taking denosumab should be considered to still have a risk of MRONJ until around nine months after their final dose. Anti-angiogenic drugs are not thought to remain in the body for extended periods of time.
Chronic systemic glucocorticoid use has been reported in some studies to increase the risk for MRONJ when taken in combination with anti-resorptive drugs20, 24–27. The combination of bisphosphonates and anti-angiogenic agents has also been associated with increased risk of MRONJ20, 28. The risk appears to be increased if the drugs are taken concurrently or if there has been a history of bisphosphonate use.
Despite these risk factors, the majority of patients are able to receive all their dental treatment in primary care, with referral only appropriate for those with delayed healing.
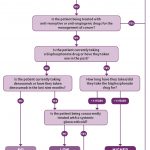
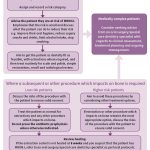
- Fig 1 – SDCEP Oral Health Management of Patients at Risk of Medication-related Osteonecrosis of the Jaw guidance
- Fig 2 – Assessment of patient risk
- Fig 3 – Managing the oral health of patients at risk of MRONJ
Guidance recommendations
The aim of the SDCEP guidance is to assist and support primary care dental teams in providing appropriate care for patients prescribed anti-resorptive or anti-angiogenic drugs and to encourage a consistent approach to their oral health management. The guidance also aims to empower dental staff to provide routine dental care for this patient group within primary care thereby minimising the need for consultation and referral to secondary care.
Risk assessment
The guidance advises practitioners to assess and record whether a patient taking anti-resorptive or anti-angiogenic drugs is at low risk or higher risk of developing MRONJ based on their medical condition, type and duration of drug therapy and any other complicating factors. An up-to-date medical history is therefore essential in identifying those patients who are, or have been, exposed to the drugs and to identify any additional risk factors, such as chronic use of systemic glucocorticoids. Careful questioning of the patient may be required, along with communication with the patient’s doctor, to obtain more information about the patient’s medical condition and drug regimen(s).
The low-risk category includes those patients who have been treated for osteoporosis or other non-malignant diseases of bone with bisphosphonates for less than five years or with denosumab and who are not taking concurrent systemic glucocorticoids. The higher risk category includes cancer patients and also those being treated for osteoporosis or other non-malignant diseases of bone who have other modifying risk factors. Figure two illustrates how risk should be assessed for each individual patient.
The risk of MRONJ should be discussed with patients but it is important that they are not discouraged from taking their medication or from undergoing dental treatment. The guidance includes details of the points which should be covered in such a discussion and patient information leaflets are also available to facilitate this dialogue. As with all patients, the risks and benefits associated with any treatment should be discussed to ensure valid consent.
Initial care
Ideally, patients should be made as dentally fit as feasible before commencement of their anti-resorptive or anti-angiogenic drug therapy. However, it is acknowledged that this may not be possible in all cases and in these situations, the aim should be to prioritise preventive care in the early stages of drug therapy. Due to their increased MRONJ risk, it is particularly important that cancer patients undergo a thorough dental assessment, with remedial dental treatment where required, prior to commencement of drug therapy. It may also be appropriate to consider consulting an oral surgery or special care dentistry specialist for advice on clinical assessment and treatment planning for these medically complex patients.
As part of this initial management, patients should be given personalised preventive advice to help them optimise their oral health. The importance of a healthy diet, maintaining excellent oral hygiene and regular dental checks should be emphasised and patients should be encouraged to stop smoking and limit their alcohol intake where appropriate. They should also be advised to report any symptoms such as exposed bone, loose teeth, non-healing sores or lesions, pus or discharge, tingling, numbness or altered sensations, pain or swelling as soon as possible.
The guidance recommends prioritising care that will reduce mucosal trauma or may help avoid future extractions or any oral surgery or procedure that may impact on bone. Radiographs should be considered to identify possible areas of infection and pathology and any remedial dental work, such as extraction of teeth of poor prognosis or treatment of periodontal disease, should be undertaken without delay. It may also be appropriate to consider prescribing high fluoride toothpaste for these patients.
Continuing care
Recommendations for continuing care advise practitioners to carry out all routine dental treatment as normal and to continue to provide personalised preventive advice in primary care. For low-risk patients, straightforward extractions and other bone-impacting treatments can be performed in primary care. A more conservative approach is advised in higher risk patients, with greater consideration of other, less invasive alternative treatment options. However, if extraction or other bone-impacting procedure remains the most appropriate course of action, these can be carried out in primary care in this patient group. There is no benefit in referring low or higher-risk patients to a specialist or to secondary care based purely on their exposure to anti-resorptive or anti-angiogenic drugs and it is likely to be in patients’ best interests to be treated wherever possible by their own GDP in familiar surroundings.
There is currently insufficient evidence to support the use of antibiotic or topical antiseptic prophylaxis specifically to reduce the risk of MRONJ following extractions or procedures that impact on bone29–32. Extraction or oral surgery sites should be reviewed, with healing expected by eight weeks. Evidence of delayed healing at eight weeks should be considered a sign of possible MRONJ. Figure three outlines the management of patients prescribed anti-resorptive or anti-angiogenic drugs.
Management of patients with suspected MRONJ
The treatment of MRONJ is beyond the scope of the guidance and patients with suspected MRONJ should be referred to a specialist in line with local protocols. Signs and symptoms of MRONJ include delayed healing following a dental extraction or other oral surgery, pain, soft tissue infection and swelling, numbness, paraesthesia or exposed bone. Patients may also complain of pain or altered sensation in the absence of exposed bone. Although the majority of cases of MRONJ occur following a dental intervention that impacts on bone, some can occur spontaneously. A history of anti-resorptive or anti-angiogenic drug use in these patients should alert practitioners to the possibility of MRONJ.
Guidance format
The main SDCEP guidance document provides practical advice and recommendations to inform the assessment of the patient’s MRONJ risk, the optimisation of their oral health during the initial phase of drug treatment and their ongoing care. A supplementary Guidance in Brief, which summarises the main recommendations, is also available.
Additional tools have been developed to support the implementation of the guidance, including patient information leaflets and information for prescribers and dispensers. The aim of the patient information leaflets is to make patients aware of the risk of MRONJ, the importance of continuing to take their medication and ways they can reduce their MRONJ risk. The leaflets provide a basis for further communication between the patient and their dentist and, ideally, should be provided to patients identified as being at risk of MRONJ at the commencement of their
drug treatment.
The guidance and the supporting documents are freely available via the SDCEP website (www.sdcep.org.uk).
Future research
MRONJ is a rare condition and consequently there is a lack of high-quality evidence on which to base guidance recommendations. High-quality research studies are required to determine the efficacy of MRONJ prevention protocols, both in the context of routine dental care and in those patents who require an extraction or procedure which impacts on bone. As an adverse drug reaction, MRONJ is monitored by the MHRA (www.mhra.gov.uk) and dental practitioners are encouraged to notify the MHRA of any suspected cases via the Yellow Card Scheme (www.yellowcard.mhra.gov.uk).
Reporting is confidential and patients should also be encouraged to report via the scheme.
It should be noted that the use of anti-angiogenic drugs in cancer is an expanding field, and it is likely that any future medications with these modes of action may also have an associated risk of MRONJ. The establishment of a national database to monitor cases of MRONJ could inform some of the research areas highlighted above and may also serve to identify other drugs which could be implicated in the disease.
As with all its guidance publications, SDCEP plans to review the recommendations in this guidance three years after publication and revise them if new evidence or experience emerges and indicates that this is appropriate.
About the author
Samantha Rutherford is a research and development manager for guidance development within the Scottish Dental Clinical Effectiveness Programme (SDCEP). She has led the development of a number of SDCEP guidance projects and was the project lead for the Oral Health Management of Patients at Risk of Medication-related Osteonecrosis of the Jaw guidance, which was published in 2017. Samantha has a PhD in medicinal chemistry and prior to her involvement in guidance development, she was a research scientist in the pharmaceutical industry.
References
1. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. Journal of Oral and Maxillofacial Surgery. 2003;61(9):1115-7.
2. Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK, McCloskey EV, et al. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. Journal of Bone and Mineral Research. 1997;12(10):1700-7.
3. MHRA. Aflibercept (Zaltrap): Minimising the risk of osteonecrosis of the jaw. Drug Safety Update. Apr 2016;9(9).
4. MHRA. Bevacizumab and sunitinib: Risk of osteonecrosis of the jaw. Drug Safety Update. Jan 2011;4(6):A1.
5. Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw -2014 update. Journal of Oral and Maxillofacial Surgery. 2014;72(10):1938-56.
6. Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. Journal of Bone and Mineral Research. 2015;30(1):3-23.
7. Kuhl S, Walter C, Acham S, Pfeffer R, Lambrecht JT. Bisphosphonate-related osteonecrosis of the jaws–a review. Oral Oncology. 2012;48(10):938-47.
8. Lee SH, Chang SS, Lee M, Chan RC, Lee CC. Risk of osteonecrosis in patients taking bisphosphonates for prevention of osteoporosis: a systematic review and meta-analysis. Osteoporosis International. 2014;25(3):1131-9.
9. Qi WX, Tang LN, He AN, Yao Y, Shen Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. International Journal of Clinical Oncology. 2014;19(2):403-10.
10. Carmona EG, Flores AG, Santamaría EL, Olea AH, Lozano MPR. Systematic Literature Review of Biphosphonates and Osteonecrosis of the Jaw in Patients With Osteoporosis. Reumatologia Clinica. 2013;9(3):172-7.
11. Grbic JT, Black DM, Lyles KW, Reid DM, Orwoll E, McClung M, et al. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. Journal of the American Dental Association. 2010;141(11):1365-70.
12. Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. Journal of the American Dental Association. 2011;142(11):1243-51.
13. Lo JC, O’Ryan FS, Gordon NP, Yang J, Hui RL, Martin D, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. Journal of Oral and Maxillofacial Surgery. 2010;68(2):243-53.
14. Amgen Ltd. Prolia 60 mg solution in a pre-filled syringe: Summary of Product Characteristics 2016. Available from:
www.medicines.org.uk/emc/medicine/23127. Accessed 14/06/17.
15. Rogers SN, Palmer NO, Lowe D, Randall C. United Kingdom nationwide study of avascular necrosis of the jaws including bisphosphonate-related necrosis. The British Journal of Oral & Maxillofacial Surgery. 2015;53(2):176-82.
16. Sammut S, Malden N, Lopes V, Ralston S. Epidemiological study of alendronate-related osteonecrosis of the jaw in the southeast of Scotland. The British Journal of Oral & Maxillofacial Surgery. 2016;54(5):501-5.
17. Solomon DH, Mercer E, Woo SB, Avorn J, Schneeweiss S, Treister N. Defining the epidemiology of bisphosphonate-associated osteonecrosis of the jaw: prior work and current challenges. Osteoporosis International. 2013;24(1):237-44.
18. Gaudin E, Seidel L, Bacevic M, Rompen E, Lambert F. Occurrence and risk indicators of medication-related osteonecrosis of the jaw after dental extraction: a systematic review and meta-analysis. Journal of Clinical Periodontology. 2015;42(10):922-32.
19. Fehm T, Beck V, Banys M, Lipp HP, Hairass M, Reinert S, et al. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): Incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecologic Oncology. 2009;112(3):605-9.
20. Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Annals of Oncology. 2012;23(5):1341-7.
21. Vahtsevanos K, Kyrgidis A, Verrou E, Katodritou E, Triaridis S, Andreadis CG, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. Journal of Clinical Oncology. 2009;27(32):5356-62.
22. Yazdi PM, Schiodt M. Dentoalveolar trauma and minor trauma as precipitating factors for medication-related osteonecrosis of the jaw (ONJ): a retrospective study of 149 consecutive patients from the Copenhagen ONJ Cohort. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2015;119(4):416-22.
23. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. The Journal of Clinical Endocrinology and Metabolism. 2011;96(4):972-80.
24. Nisi M, La Ferla F, Karapetsa D, Gennai S, Miccoli M, Baggiani A, et al. Risk factors influencing BRONJ staging in patients receiving intravenous bisphosphonates: a multivariate analysis. International Journal of Oral and Maxillofacial Surgery. 2015;44(5):586-91.
25. Otto S, Troltzsch M, Jambrovic V, Panya S, Probst F, Ristow O, et al. Tooth extraction in patients receiving oral or intravenous bisphosphonate administration: A trigger for BRONJ development? Journal of Cranio-maxillo-facial Surgery. 2015;43(6):847-54.
26. Taylor T, Bryant C, Popat S. A study of 225 patients on bisphosphonates presenting to the bisphosphonate clinic at King’s College Hospital. British Dental Journal. 2013;214(7):E18.
27. Tsao C, Darby I, Ebeling PR, Walsh K, O’Brien-Simpson N, Reynolds E, et al. Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. Journal of Oral and Maxillofacial Surgery. 2013;71(8):1360-6.
28. Guarneri V, Miles D, Robert N, Dieras V, Glaspy J, Smith I, et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Research and Treatment. 2010;122(1):181-8.
29. Ferlito S, Puzzo S, Liardo C. Preventive protocol for tooth extractions in patients treated with zoledronate: a case series. Journal of Oral and Maxillofacial Surgery. 2011;69(6):e1-4.
30. Lodi G, Sardella A, Salis A, Demarosi F, Tarozzi M, Carrassi A. Tooth extraction in patients taking intravenous bisphosphonates: a preventive protocol and case series. Journal of Oral and Maxillofacial Surgery. 2010;68(1):107-10.
31. Mozzati M, Arata V, Gallesio G. Tooth extraction in osteoporotic patients taking oral bisphosphonates. Osteoporosis International. 2013;24(5):1707-12.
32. Schubert M, Klatte I, Linek W, Muller B, Doring K, Eckelt U, et al. The saxon bisphosphonate register – therapy and prevention of bisphosphonate-related osteonecrosis of the jaws. Oral Oncology. 2012;48(4):349-54.
A visitor to the biennial International Dental Show (IDS) held in Cologne in March this year could have been mistaken for thinking that they were attending an engineering trade show rather than a dental event. The number of companies displaying milling machines, 3D printers, CAD-CAM hardware and software reflected the rapid developments in digital dentistry in recent years.
There is no area of dentistry that is not being ‘disrupted’ by the move from traditional analogue to digital practice, and implant dentistry is no exception. Planning software has been available for many years, but the recent addition of two concepts has seen a renewed interest in guided surgery. This article will examine optical scanning and desktop 3D printing in the production of drill guides, that may facilitate accurate surgical technique benefiting both the clinician and patient.
The aim of a detailed pre-surgical assessment is to ensure that the implant is placed in an optimal position so that it may be restored to achieve the desired aesthetic and functional result. Clinical and radiographic examination of the patient is required before implant placement. The assessment should consist of visual examination, palpation of superficial structures and the measurement of gap width, crest width and maxillo-mandibular relationships.
Radiographs are used during implant treatment planning and Cone beam computer tomography (CBCT) is used to view a 3D image of hard tissue. This enables the clinician to measure the height and width of the ridge and assess the quality of the planned implant site and adjacent anatomical structures. While there is still a place for conventional 2D imaging techniques, the use of a CBCT scan is now considered to be standard practice in implant dentistry1,2.
The images from CBCT scans may be imported into many software packages to allow the clinician to plan the implant placement in a virtual environment prior to surgery. Programmes such as Simplant (Dentsply Implants) have had the ability to not just plan implant placement, but to use CAD (computer aided design) output to CAM (computer aided manufacture) to produce drill guides for many years. Thus, it is important to state that neither the use of CBCT or guided surgery is new.
The use of guided surgery has previously been restricted by two factors:
- Inability of CBCT to provide intra-oral soft tissue images
- Cost of drill guide manufacture.
Intra-oral scanning provides detailed surface images of the dental hard and soft tissue. These images may be merged with hard-tissue images from a CBCT scan to provide a 3D view of teeth/bone and of soft issue. As with previous software, CAD planning allows the manufacture of drill guides. The availability of affordable 3D printers now allows the dentist to produce in-house accurate and cost-effective drill guides.
Both intra-oral scanning and 3D printing date back to the 1980s. Nearly 30 years later, both are changing the way we plan implant dentistry.
Optical intra-oral scanner
CEREC 1 was developed by Dr Werner H Mormann and Dr Marco Brandestini at the University of Zurich. Thanks to more powerful (and smaller) computers and the developing application of CAD/CAM systems at the time, their vision resulted in the first of the series of CEREC machines. From its early beginnings, limited to milling ceramic inlays, optical scanning and CAD/CAM manufacture are today at the focus of many developments in restorative and
surgical dentistry.
Optical scanners work by directing a light beam at an object, the light beam is bounced back to the scanner and the image is digitised. A digital image is a series of triangles joined together to make a 3D image – a meshwork of triangles – stored in digital format.
STL (STereoLithography) is the file format of stereolithography CAD software that is widely used for rapid prototyping, 3D printing and computer-aided manufacturing. Images from dental optical scanners may output to STL files (open platform) or a scanner specific file format (closed platform). The two market-leading intra-oral scanners, Trios (3Shape, Denmark) and CEREC (Dentsply Sirona, Germany) have both recently enabled STL file output for their respective scanners. This move to STL open-platform enables dentists and dental labs to make use of the STL files in a wide range of software packages, rather than being restricted to a closed-platform programme.


In relation to implant planning software, the STL file is imported and combined with DICOM (Digital Imaging and Communication in Medicine) data from the CBCT scan (Fig 1). Many planning programmes are available – a selection is listed in Table one. A comparison of the strength and weaknesses of each is outwith the scope of this article but, as with any software, one should assess its ease of use, support, cost, open/closed platform.
The software allows the dentist to plan the implant position (Fig 2) and then design a drill guide (Fig 3). The design is outputted to an STL file, which can then be sent to a 3D printer either on-site or to a third party e.g. a dental lab.
- Figure 1
- Figure 2
- Figure 3
- Figure 4
- Figure 5
- Figure 6
- Figure 7
- Figure 8
3D printing
Early additive manufacturing equipment and materials were developed in the 1980s. 3D printing, also known as additive manufacturing or rapid prototyping, refers to processes used to create a three-dimensional object in which layers of material are formed under computer control to create an object. 3D printing builds a three-dimensional object from a CAD model by successively adding material layer by layer.
An early use of 3D printing in dentistry was shown in 1984 when a team working on sub-periosteal implants at Loma Linda University in California used 3D printing to produce a mandible from a CT scan3. The printed model of the mandible was then used to manufacture the sub-periosteal implant. The CT scan and 3D print removed the need for a surgical visit to take an impression of
the mandible.
The size and cost of 3D printers has fallen, while the range of 3D printers continues to grow at an astonishing pace. Originally aimed at industrial manufacturers, 3D printers are now available for all, from those aimed at the domestic hobbyist, to small and medium-sized business and large-scale operations.
In recent years, dental applications in desktop 3D printing have rapidly taken off. FormLabs (Massachusetts, US) products have been at the forefront of dental innovation. Aimed at the “prosumer” (production by consumer) market, dentists and dental labs now use the Form 2 (Fig 4) to create surgical guides, study models, bleaching trays, retainers, aligners, and with the imminent launch of FormLab denture resin, it will soon be possible to 3D print dentures. The Form 2 printer has a range of dental resins detailed in Table two.
The dental SG resin is an autoclavable, class 1 medical device directive registered biocompatible resin. Dental SG is used for printing surgical guides. It was launched by FormLabs in 2016 and was the first biocompatible resin for desktop 3D printing. Following 3D printing in the Form 2, dental SG is light cured in a light oven (Fig 5) (Brelux Power Unit 2, Bredent GmbH, Germany), the appropriate drill sleeve is selected and fitted for either a pilot of fully guided surgery (Fig 6). The drill guide may then be autoclaved prior to surgery.
Direct printing of surgical guides has traditionally required large-scale 3D printers that are beyond the expense of most dental laboratories and practices. The introduction of dental SG Resin and the Form 2 allows for surgical guide printing in dental practices and smaller dental labs.
Discussion
It has previously been possible to combine intra-oral detail with a CBCT scan without using an optical scanner. This was achieved using a dual scan technique, whereby a CBCT scan of a study model provided surface hard and soft tissue detail that could be merged with a CBCT scan. In edentulous cases where there is no intra oral hard tissue, it is not possible to use optical scans as intra-oral soft tissue cannot be linked to a CBCT scan. In such cases it is still necessary to use a dual scan technique with radiopaque markers in a denture. Thus, merging intra-oral and CBCT scans is restricted to dentate and partially edentulous cases.
The case for the use of drill guides is that they increase the accuracy of implant placement. Many studies have evaluated the accuracy of guided surgery4,5,6. There is a perception from the marketing of guided surgery by implant companies that this technique is easier than non-guided surgery. In many cases this may be correct, but it is essential that anyone undertaking guided surgery has a sound understanding of implant case planning and surgical protocols. The guide does not place the implant, the surgeon places the implant. The surgeon must be familiar with conventional approaches to implant placement and bone generation techniques before embarking on guided surgery.
It is important to state that guided surgery does not mean flapless surgery. 3D printed guides may be used for the following:
- As pilot guides only with an appropriate muco-periosteal flap
- Fully guided with an an appropriate muco-periosteal flap
- Fully guided with a flap-less technique (Fig 7).
The surgical flap (or lack thereof) will be determined by the need for augmentation at the time of surgery. Flap-less surgery should be restricted to cases with ample bone volume and no need for either soft or hard tissue augmentation. Where there is any doubt with regard for the need for augmentation, an appropriately designed flap should be raised. Where suitable, the benefits of flap-less surgery with a drill guide include: reduced surgery time, minimal post operative complications, no sutures.
Drill sleeves for pilot guides will be determined by the diameter of the pilot drill, with a range of different sizes that are fitted to the guide following 3D printing. Drill sleeves for fully guided surgery are specific to the implant system being used. The surgeon should have additional training in the use of the guided surgical kit. This differs from the standard surgical kit in that each drill will have a stop to determine the depth of the osteotomy relative to the drill sleeve.
The drill is designed so that there is an offset between the drill sleeve and depth of the planned osteotomy (Fig 8). Inner sleeves or keys are used to match the diameter to the corresponding osteotomy drill (Fig 6). Each step of guided surgery is detailed on a drilling protocol supplied with the treatment plan.
The surgeon placing the dental implant is responsible for the implant placement, not the drill guide. The treatment plan for guided surgery must be approved by the implant dentist prior to 3D printing. When the dental surgeon is the dentist planning the guide, this is straightforward. In situations whereby the CBCT scan/implant planning is referred to a third party, then it is essential that there is effective communication between the parties and that the guided plan is approved by the referring dentist, who is ultimately responsible for the implant placement.
Clinical records including intra-oral images from an optical scanner, CBCT scan, treatment plan and drill guide demonstrate a high standard of planning and record keeping. Such documentation may be of benefit for medico-legal purposes. As with CBCT scans, it may be that this approach will become the gold standard of implant treatment planning and record keeping.
Conclusion
It seems that all of the pieces of the guided surgery jigsaw puzzle have now fallen into place: CBCT, optical scanning, CAD/CAM software and desktop 3D printing. Digital techniques, both hardware and software, are changing the way that dentistry is practised with benefits for both dentists and our patients. Implant companies are promoting guided surgery and it is essential that dentists are trained in such techniques and have a full understanding of the benefits and limitations of guided surgery. At the end of the day, computers and robots don’t place the implants… not yet, at least.
About the authors
Michael Dhesi is a GDP at Clyde Dental Centre. He qualified in 2012 with BDS(Hons) from the University of Glasgow and has subsequently completed MFDS RCPS(Glasg) and an MSc in Advanced General Dental Practice at the University of Birmingham. Michael’s focus is in minimally invasive and adhesive restorative dentistry.
He also has interests in the management of dental anxiety and oral surgery and welcomes referrals in these areas.
Clive Schmulian qualified from Glasgow University in 1993. Throughout his time in general dental practice, he has developed his clinical skills by obtaining a range of postgraduate qualifications, which in turn led him to develop an interest in digital imaging in both surgical and restorative dentistry. He is a director of Clyde Munro.
References
1. Bornstein MM, Scarfe WC, Vaughn VM, Jacobs R. Cone beam computed tomography in implant dentistry: a systematic review focusing on guidelines, indications, and radiation dose risks. Int J Oral Maxillofac Implants. 2014;29 Suppl:55-77.
2. Jacobs R, Quirynen M. Dental cone beam computed tomography: justification for use in planning oral implant placement. Periodontol 2000. 2014 Oct;66(1):203-13.
3. Truitt HP1, James R, Altman A, Boyne P. Use of computer tomography in subperiosteal implant therapy. J Prosthet Dent. 1988 Apr;59(4):474-7.
4. Raico Gallardo, da Silva-Olivio, Mukai, Morimoto, Sesma, Cordaro L. Accuracy comparison of guided surgery for dental implants according to the tissue of support: a systematic review and meta-analysis. Clin Oral Implants Res. 2017 May;28(5):602-612.
5. Ángeles Fernández-Gil, MD/Herminia Serrano Gil, MD/Miguel González Velasco, PhD/José C. Moreno Vázquez, MD, PhD. An In Vitro Model to Evaluate the Accuracy of Guided Implant Placement Based on the Surgeon’s Experience. JOMI. Volume 32, Issue 3 May/June 2017 Pages 515–524.
6. Corina Marilena Cristache, Silviu Gurbanescu. Accuracy Evaluation of a Stereolithographic Surgical Template for Dental Implant Insertion Using 3D Superimposition Protocol. Int J Dent. 2017; 2017.
The anatomy of the apical foramen changes with age as root formation has yet to be completed when teeth erupt. The completion of root development and closure of the apex occurs up to three years after eruption1.
Patients who present with immature apical formation (see Fig 1) pose a challenge due to the presence of large open apices along with divergent and thin dentinal walls that are susceptible to fracture. Historically, we have tried to generate formation of an apical barrier by repeated placement of calcium hydroxide over many months, or more recently by immediate barrier formation with a Mineral Trioxide Aggregate (MTA) plug.
Ideal management would involve regeneration of new pulpal tissue and continued root formation. Novel techniques for dealing with immature apices such as apexogenesis sometimes claim to be regenerative techniques. However, assessment of the composition of this regenerated tissue has proven to be difficult and it seems that it is made up of periodontal and bone tissue rather than tissue of pulpal origin2. As clinicians we need to consider whether this is better than formation of an apical barrier and obturation by conventional means?
Root development (see Table 1)
Classically, there are two types of open apices; blunderbuss and non-blunderbuss. In the former, the walls of the canal are divergent and flaring, the apex is funnel shaped and typically wider than the coronal aspect of the canal. In a non-blunderbuss apex, the walls of the canal may be parallel to slightly convergent. The apex, therefore can be broad shaped or convergent.
What are the causes of open apices?
Incomplete root development often arises secondary to pulpal necrosis arising as a result of caries or trauma. Both foraminal and peri-foraminal resorption of the root end may also arise in the presence of a periapical lesion4. This may alter the anatomy of a pre-existing open apex further. Iatrogenic enlargement of the root end may also arise due to poor control of working length and subsequent enlargement with both hand and rotary files.
What problems are faced clinically?
Teeth with open apices often tend to have thin dentinal walls that are susceptible to fracture before, during or after endodontic treatment. Frequently, they present with periapical lesions, which may or may not be associated with apical resorption. Short roots compromise the crown-root ratio, often affecting long-term prognosis.
Fractures of the crown are common following trauma. This can compromise aesthetics, especially in the anterior region, and there may be a lack of tooth tissue present. In long-standing cases these teeth may also undergo discolouration. Large open apices pose a challenge in determining the working length, decision on the necessity of root canal preparation, and achieving control
during obturation.
- Fig 1a
- Fig 1b
- Fig 1c
- Fig 1d
- Fig 2
- Fig 3
- Fig 4a
- Fig 4b
- Fig 4c
- Fig 4d
- Fig 4e
How is the working length determined?
There is relatively little data regarding the value of radiography and electronic apex locator (EAL) use when root formation is incomplete, and supplementary measurement techniques may be helpful. When using an EAL to measure working length in such cases, it is essential to use a file which is well matched to the apical size (see Fig 2) where possible. The paper point technique described by Rosenberg to supplement initial apex locator readings could be considered for the working length determination of open apices in relatively straight canals5.
Marcos-Arenal et al.6 in an in vivo study, demonstrated an 87 per cent accuracy of this technique in establishing working length to within 0.5mm of the apical foramen. While El Ayouti et al.7 proposed a tactile method involving the use of a size 25 K-file bent at the tip, with its orientation marked with a silicone ring. The file was bent to facilitate ease of use. In this study, 95 per cent of cases were accurate to within 0.5mm of the apical foramen.
Do I need to instrument the canal?
During conventional root canal treatment, the role of instrumentation is to achieve removal of vital and necrotic tissues from the root canal system, along with infected root dentine8. It aims to prepare the canal space to facilitate attempts at disinfection using irrigants and medicaments. As a result, minimal instrumentation of teeth with open apices (and thin dentinal walls) is needed due to the ease in placement of irrigation devices close to the working length.
How do I obturate the canal?
The options for obturation are dependent on whether or not we aim to create an apical barrier. Figure 3 highlights the different options. Apexification and apexogenesis are two endodontic procedures which attempt to either induce apical repair by initiating a hard tissue barrier across an open apex or to promote the continued formation of the apical portion of the root9.
Apexification
Calcium Hydroxide has been the first choice material for apexification. Placement and repeated changes over the course of five to 20 months induces the formation of a calcific barrier. The unpredictable and lengthy course of treatment presented challenges, particularly as it required a high level of patient compliance. For this reason, one visit apexification has been suggested.
MTA has been proposed as a material suitable for one visit apexification as it combines a bacteriostatic action, biocompatibility and a favourable sealing ability. Placement of a 3mm thickness of MTA in the apical portion of an ‘open apex’ permits the vertical condensation of warm gutta percha into the remainder of the canal (See Fig 4).
Apexogenesis
Case reports in the literature over the past 10 years have demonstrated successful revascularisation and regeneration of immature permanent teeth with apical periodontitis. Banchs and Trope10 irrigated necrotic teeth with sodium hypochlorite, and placed an antibiotic tri-paste dressing consisting of metronidazole, minocycline and ciprofloxacin. Four weeks later, the tooth was re-accessed and bleeding encouraged at the apex, allowing a clot to form 3mm below the CEJ. MTA was placed on the blood clot and the tooth restored with composite. Follow-up radiographs demonstrated complete apical healing and continued root formation.
Shin et al.11 treated a non-vital mandibular second premolar tooth using irrigation with 6 per cent sodium hypochlorite and 2 per cent chlorhexidine without instrumentation in a single visit. The successful outcome of this case report suggests that this conservative revascularisation treatment approach can create a suitable environment for pulpal repair, resulting in the completion of root maturation.
McCabe12 recently published a case report showing disinfection with 5 per cent sodium hypochlorite followed by the induction of a blood clot into the root canal space may be sufficient to promote revascularisation in certain circumstances using a single visit protocol.
Most of the case reports regarding apexogenesis as a treatment modality have shown an increase in dentinal wall thickening and root length, with a reduction in the volume of the pulp canal space visible radiographically. Histological analysis of teeth which had undergone revascularisation treatment demonstrated that the mineralised layer on the walls which was present appeared to be of periodontal origin rather than pulpal origin12.
What does the future hold?
It appears that current treatment approaches tend to stimulate reparative rather than regenerative responses in respect of the new tissue generated, which often does not closely resemble the physiological structure of dentine-pulp complex. Although patients requiring treatment undoubtedly make up a small proportion of our patients, and despite the biological limitations, such techniques appear to offer significant promise for improved treatment outcomes2.
The main question is whether our patients are better served by apexification and formation of an apical barrier via an MTA plug, or whether apexogenesis and generation of reparative tissue within the canal space, even if it is periodontal in origin, is better? It could be argued that apexogenesis will make the tooth more suitable for restoration, as teeth which have undergone apexification tend to be more fragile and prone to cervical fractures.
Any attempt to undergo biological healing should prove to be more beneficial in the long term. Further research is required into this novel approach to apexogenesis to assess the long-term prognosis of these teeth. Current research on pulp regeneration is growing and provides exciting possibilities for greater biological approaches to endodontics in the future13.
In the first part of this article we looked at the medical and dental history, radiographic examination and diagnosis of a 49-year-old male patient who initially attended for an emergency appointment. He was experiencing pain and swelling from LL4, which had previously been root treated while on holiday.
Treatment Plan
Since the patient was well motivated and wished for a predictable long-term solution, he opted for reduction of the infection with root canal retreatment under private contract. He did not wish to keep the tooth under observation since there was a risk of his symptoms returning and he did not wish for extraction of tooth LL4 since this would have functional and aesthetic implications for him.
The treatment procedures with risks were discussed at length. The patient was advised that treatment success would be achieved by removing all of the previous obturation materials, locating the additional canal(s), chemically and mechanically disinfecting the canals and finally obturating the canals and providing a definitive coronal seal.
The patient was given the opportunity to ask questions and these were answered to the patient’s satisfaction. The patient was given a good prognosis upon successful completion of treatment with a success rate approximated at 62-86 per cent, based on evidence published by Sjogren et al 1990. The patient was advised that non-surgical root canal retreatment was indicated as a primary treatment option as indicated by Rahbaran et al 2001. Consent was taken and two subsequent appointments made.
Treatment plan was as follows:
a) Antibiotic prescription to reduce facial swelling.
b) Oral hygiene advice and instruction plus interdental cleaning demonstration.
c) Scale and polish
d) Restorability assessment and root canal re-treatment of tooth LL4 over two visits
e) Definitive composite restoration of access cavity
f) Review (one year).
Items b and c were performed by the practice
dental hygienist.
- Fig 1 – Previous GP removed
- Fig 2 – LL4 WL radiograph of buccal canal
- Fig 3 – LL4 WL radiograph of lingual canal
- Fig 4 – LL4 MAC radiograph
- Fig 5 – LL4 final radiograph
- Fig 6 – LL4 one-year review radiograph
Treatment description
After the patient was questioned about his lack of any allergies, a prescription for amoxicillin 500mgs tds for five days was given as per recommendations by SDCEP (Scottish Dental Clinical Effectiveness Programme) 2014.
The patient attended when he could schedule an appointment around his work commitments, which was 16 days later. The restorability of tooth LL4 was discussed and, since the present restoration was well fitting and functioning well, it was decided to maintain this onlay and perform the root canal retreatment through the occlusal surface. This decision was based on evidence published by Saunders and Saunders 1994. The patient, however, was warned that in the event that the onlay suffered damage as a result of the access cavity, he would require a new restoration. The possibility of a future full coverage crown was also discussed. The procedure was again explained and the patient consented to treatment.
Topical anaesthesia was administered with 20 per cent benzocaine. After 60 seconds, as recommended by Malamed 1990, a buccal and lingual infiltration was administered with Septanest 2.2mls articaine hydrochloride 4 per cent with adrenaline 1:100,000. An articaine infiltration was chosen as opposed to a 2 per cent lignocaine or 3 per cent prilocaine inferior dental block since, in the author’s experience, articaine gives fast and profound anaesthesia which is ideal for root canal treatments of lower premolars, as recommended by Malamed et al 2000. Rubber dam (Optidam, Kerr) was then placed and sealed with oraseal to prevent any liquid leakage into the oral cavity.
An access cavity was prepared with Dentsply Access Cavity burs, through the existing composite onlay with the aid of X2 magnification surgical loupes. The obturation material was located buccally and identified as pink gutta percha. This was subsequently removed with the aid of Gates Glidden burs, Hedstrom stainless steel files sizes 25 and 30 and solvent (chloroform). This solvent was used since Tamse et al 1986 showed chloroform to be an effective solvent for the purposes of gutta percha removal. Also, Chutich et al 1998 advised chloroform poses minimal risks to the operator and patient when used in retreatment cases. Figure 1 shows a piece of gutta percha removed.
The canal was irrigated copiously with 5.25 per cent heated (50 degrees celsius) sodium hypochlorite. Heated sodium hypochlorite was used because research by Cunningham et al 1980, Abou-Rass et al 1981, Gambarini et al 1998 and Sirtes et al 2005 have all shown the enhanced tissue dissolving effects of 5.25 per cent sodium hypochlorite when heated to 45-60°C. A radiograph was then taken to verify the working length (20mm from the occlusal surface reference point) and to confirm gutta percha removal since a zero reading had been achieved (at 20.5mm and 0.5mm deducted to give the correct working length) with an electronic apex locator (EAL) (Morital ZX). The EAL was used since much published research appears to have the aforementioned EAL model as being the gold standard amongst other EAL’s. Kobayashi et al 1995 discussed the accuracy of EAL’s of measuring the working length to 0.5mm of the apical foramen. Many studies have concurred with this study. Patency had also been achieved in the buccalcanal (Fig 2).
The tooth was then assessed with the aid of a surgical operating microscope (Carl Zeiss Pico) at x10 magnification. The remaining gutta percha was removed with the aid of Gooseneck burs and a DG16 probe. To locate the lingual canal which was apparent on the initial radiograph, root dentine was removed with ultrasonics (Satalec P5, Satalec-Acteon Merignac, France) using a Start X size three tip and K1 carbon file (Sybron Endo) used to enter the canal. A working length was established as before and measured with an EAL and verified with another radiograph. The lingual canal was again patent and had a working length of 19mm from the occlusal surface reference point (Fig 3).
GG burs were again used to achieve straight-line access being careful not to remove excessive dentine and restoration material. Copious irrigation with 5.25 per cent heated sodium hypochlorite was performed and a glide path was established with 2 per cent taper K files, 10-20 with patency being confirmed by recapitulating with a size 10 stainless steel
K file. The files were used with a watch-winding action and finished with a push-pull motion until a smooth glide path was created.
Canals were dried and a temporary dressing of calcium hydroxide paste (Ultracal ultradent USA) placed in each canal with 1mm of the WL. Several studies including Mohammadi et al 2011, Siqueira et al 1999 and Sjogren et al 1991 have shown and discussed the antimicrobial benefits of calcium hydroxide as an inter-appointment medicament and have recommended its use for between two and four weeks. A foam pellet (Roeko) was placed in the pulp chamber since this is easily removed and does not have fibres which may lodge into a root canal space unlike cotton wool. A GI temporary restoration was placed in the access cavity. POIG and the patient made an appointment for two weeks later.
At the second appointment the patient reported tooth LL4 to be completely symptom-free. Upon clinical examination, no submandibular swelling was noted, no buccal or lingual swelling noted, the tooth was not TTP and the temporary dressing was in situ and well-sealed.
Topical anesthetic was placed as before and septanest LA was administered as before. Rubber dam with oraseal was placed and the temporary dressing removed. The canals were irrigated with 5.25 per cent heated NaOCl and, after gauging the canal dimensions with size 20 and 25 K files, were prepared with Reciproc R25 reciprocating rotary file (tip diameter of 0.25mm with a 8 per cent taper) using a X-Smart Plus Endodontic motor (Dentsply). The Reciproc rotary file system was chosen to ensure complete residual gutta percha removal, efficient infected root dentine removal whilst maintaining the integrity of the root canal space. This file has M-Wire technology which has been shown to have greater flexibility and resist cyclic fatigue fracture. It has an S-shaped cross-section which enhances dentine removal from the root canal and a regressive taper. Research by Yared 2008, Yared 2011 and Gavini et al 2012 have shown this file to have superior properties when compared to a continuous motion NiTi file system and has been shown to be particularly efficient when used in retreatment cases.
Both canals were prepared to length with a pecking motion as per the manufacturers guidelines. A MAC radiograph was taken to verify GP lengths after gauging each canal to an apical width size 25 (MAF) (Fig 4).
A final rinse was performed with 5.25 per cent heated NaOCl with sonic agitation using a Waterpik flosser for 30 seconds in each canal. This device uses sonic energy to vibrate a polymer tip which does not damage the root dentine if touched. It works at the same frequency and principle as the Endoactivator which Ruddle 2007, Ruddle 2008, Mancini et al 2013 and Paragliola et al 2010 have shown enhances smear layer removal and the effectiveness of the final rinse protocol. Each canal was again irrigated with NaOCl and then irrigated with 17 per cent EDTA (as recommended by Bystrom et al 1985 and Calt et al 2002) for a further 30 seconds with a final flush with NaOCl. The canals were then dried with R25 paper points until the tips of the points were withdrawn from the canals dry.
Obturation was performed with R25 gutta percha cones sealed with AH Plus sealer via continuous wave and heated backfill. B&L (alpha and beta) hot-tip and GP gun were used to facilitate this. AH Plus sealer has been shown to have excellent sealing qualities as described by Ungor et al 2006 and Siqueira et al 2000. The heated GP was condensed with a size two Machtou plugger. A heated obturation method was chosen to achieve a 3 dimensional seal as advised by Schilder 1967.
The continuous wave method of root canal oburation has been shown to be an efficient method to achieve a homogenous gutta percha seal as described by Buchanan 1994 and 1996. Gooseneck burs were then used to level and smooth the GP to the canal orifice level. The chamber was cleansed with isopropyl alcohol and sealed with SDR light-cured flowable composite after applying 37 per cent hydrophosphoric acid for 20 seconds, washing with water for 20 seconds, drying with air and applying a dentine bonding agent (Prime and Bond NT). The remainder of the access cavity was restored with composite resin (Filtek Supreme A3) and light cured for 20 seconds.
The rubber dam was then removed, the occlusion checked in ICP and lateral excursions and the surface polished with rubber cups and diamond polishing paste. A final radiograph was taken showing a well-condensed obturation with good coronal, mid and apical seal. Some sealer extrusion was noted (Fig 5).
Review
At a one-year review appointment the patient reported no symptoms and he was delighted at how quickly the tooth had settled post-treatment. Clinical examination reported no significant findings (no soft tissue swelling or tenderness to percussion). The radiograph taken (Fig 6) shows signs of radiographic healing since the radiolucency visible on the pre-operative radiograph is not present. Interestingly, there appears to be some sealer present mid canal on either one or both canals. This may be a lateral canal(s) which was not evident from the initial post-op radiograph taken a year ago. Again, some sealer extrusion is noted in the lingual canal. The definitive restoration was also functioning well.
Discussion
Root canal treatment can be a very challenging dental discipline due to the complexity of the root canal system. Root canal retreatment adds another dimension because the clinician has to dismantle another clinician’s work and orientate himself according to the radiographic evidence available. Fortunately, in this case, the radiographic view available was sufficient to obtain the necessary canal anatomy. Upon reflection, another 5 degree angled- view peri-apical radiograph may have given a better image of the additional root. The decision was made, however, according to IRMER(2000) and IRR99, not to expose the patient to further radiation and that the available radiograph was sufficient for diagnostic purposes.
This case also demonstrates the importance of using a surgical operating microscope (SOM). As discussed by Vertucci 1984 and 2005, mandibular permanent first premolars can present with a number of canal configurations. In this case a class V configuration is noted and according to Vertucci 1984, approximately 24 per cent of mandibular lower first premolars have this configuration. The lingual canal could be seen with the SOM but was not visible with x2 magnification surgical loupes. The SOM certainly enhanced the operative procedure and enabled me to treat this case more effectively and with greater precision.
In every endodontic case I complete, I feel it important to reflect and identify where the treatment could have been improved. In this case a radiograph taken after placement of Ca(OH)2 would have shown me the position of the radio-opaque paste and given me confirmation that it was placed within 1-2mm of the radiographic apex in the apical third of the root canals. Also, a radiograph taken as a midfill would have confirmed that the apical third had been obturated adequately before the heated backfill obturation was commenced. Since obtaining a good apical seal is paramount, a customised MAC possibly could have been used to achieve the apical anatomical detail with the use of
chloroform solvent.
This method, however, was not used since the heated method of obturation used enabled an accurate apical seal. Fortunately the final radiograph shows a well compacted obturation in both canals at the correct working length. A criticism of the final radiograph would be that there appears to be a small void above the GP where the SDR has been placed. This will be kept under observation and since the remainder of the composite restoration is well condensed, I see no reason to replace the composite at this time. There is also some sealer extrusion but according to Sari et al 2007 and Augsburger and Peters 1990, no consequences should result and no foreign body reaction should ensue. Periapical healing is not impaired in adults. Sari et al 2007 also showed that approximately 56 per cent of extruded AH Plus disappears over a 4 year period. The patient has been advised and this will be reviewed, although not with a radiograph due to limiting the patient to x radiation, at every subsequent examination.
The one-year post-operative radiograph shows signs of radiographic healing. There are, of course, limitations of this imaging modality. Radiographs give a two-dimensional view of a three-dimensional spatial relationship. Although there appears to be radiographic healing, the image does not show all of the surfaces of this tooth’s apex. Cone beam computed tomography (CBCT) could give a more detailed and accurate view of all the tooth’s surfaces and allow a more definitive conclusion to be made with regards to the healed status of the tooth. Ultimately, histological analysis would provide a definitive answer but this is not possible.
Since the patient reports that the tooth is asymptomatic, taking a CBCT image would not be recommended and is not indicated according to current European Society of Endodontology 2014 guidelines. According to Patel et al 2015, teeth that appear radiographically as healed may be diagnosed as having a radiolucent periapical area on a CBCT. This may be present in an asymptomatic tooth and raises the question of what should be done to manage this if the patient is not experiencing any symptoms.
References
Abou-Rass M and Oglesby SW, 1981. The effects of temperature, concentration, and tissue type on the solvent ability of sodium hypochlorite. Journal of endodontics, 7(8), pp. 376-377.
Augsburger, RA and Peters, DD, 1990. Radiographic evaluation of extruded obturation materials. Journal of endodontics, 16(10), pp. 492-497.
Buchanan LS, 1996. The continuous wave of obturation technique:’centered’condensation of warm gutta percha in 12 seconds. Dentistry today, 15(1), pp. 7.
Buchanan LS, 1994. The continuous wave of condensation technique: a convergence of conceptual and procedural advances in obturation. Dentistry today, 13(10), pp. 5.
Bystrm A and Sunvqvist G, 1985. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. International endodontic journal, 18(1), pp. 35-40.
Calt S and Serper A, 2002. Time-dependent effects of EDTA on dentin structures. Journal of endodontics, 28(1), pp. 17-19.
Chutich MJ, Kaminski EJ, Miller DA and Lautenschlager EP, 1998. Risk assessment of the toxicity of solvents of gutta-percha used in endodontic retreatment. Journal of endodontics, 24(4), pp. 213-216.
Cunningham WT and Joseph SW, 1980. Effect of temperature on the bactericidal action of sodium hypochlorite endodontic irrigant. Oral Surgery, Oral Medicine, Oral Pathology, 50(6), pp. 569-571.
Dalton BC, Rstavik D, Phillips C, Pettiette M and Trope M, 1998. Bacterial reduction with nickel-titanium rotary instrumentation. Journal of endodontics, 24(11), pp. 763-767.
Gambarini G, De Luca M and Gerosa R, 1998. Chemical stability of heated sodium hypochlorite endodontic irrigants. Journal of endodontics, 24(6), pp. 432-434.
Kakehashi S, Stanley HR and Fitzgerald RJ, 1965. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surgery, Oral Medicine, Oral Pathology, 20(3), pp. 340-349.
Kobayashi C, 1995. Electronic canal length measurement. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 79(2), pp. 226-231.
Malamed SF, 2014. Handbook of local anesthesia. Elsevier Health Sciences.
Malamed SF, Gagnon S and Leblanc D, 2000. Efficacy of articaine: a new amide local anesthetic. The Journal of the American Dental Association, 131(5), pp. 635-642.
Mancini M, Cerroni L, Iorio L, Armellin E, Conte G and Cianconi L, 2013. Smear layer removal and canal cleanliness using different irrigation systems (EndoActivator, EndoVac, and passive ultrasonic irrigation): field emission scanning electron microscopic evaluation in an in vitro study. Journal of endodontics, 39(11), pp. 1456-1460.
Mohammadi Z and Dummer PMH, 2011. Properties and applications of calcium hydroxide in endodontics and dental traumatology. International endodontic journal, 44(8), pp. 697-730.
Molander, A., Reit, C. And Dahln, G., 1999. The antimicrobial effect of calcium hydroxide in root canals pretreated with 5 per cent iodine potassium iodide. Dental Traumatology, 15(5), pp. 205-209.
Nair PR, Sjgren U, Figdor D and Sundqvist G, 1999. Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 87(5), pp. 617-627.
Nair PR, Sjgren U, Krey G, Kahnberg K and Sundqvist G, 1990. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. Journal of endodontics, 16(12), pp. 580-588.
Paragliola R, Franco V, Fabiani C, Mazzoni A, Nato F, Tay FR, Breschi L and Grandini S, 2010. Final rinse optimization: influence of different agitation protocols.Journal of endodontics, 36(2), pp. 282-285.
Patel S, Durack C, Abella F, Shemesh H, Roig M and Lemberg K, 2015. Cone beam computed tomography in endodontics–a review. International endodontic journal, 48(1), pp. 3-15.
Patel S, Durack C, Abella F, Roig M, Shemesh H, Lambrechts P and Lemberg K, 2014. European Society of Endodontology position statement: The use of CBCT in Endodontics. International endodontic journal, 47(6), pp. 502-504.
Rahbaran S, Gilthorpe MS, Harrison SD and Gulabivala K, 2001. Comparison of clinical outcome of periapical surgery in endodontic and oral surgery units of a teaching dental hospital: a retrospective study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 91(6), pp. 700-709.
Ruddle CJ, 2008. Endodontic Disinfection. Endodontic practice, .
Ruddle CJ, 2007. Hydrodynamic disinfection. Dentistry today.
Sari Ş and Durutűrk L, 2007. Radiographic evaluation of periapical healing of permanent teeth with periapical lesions after extrusion of AH Plus sealer. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 104(3), pp. e59.
Saunders WP and Saunders EM, 1994. Coronal leakage as a cause of failure in root‐canal therapy: a review. Dental Traumatology, 10(3), pp. 105-108.
Schilder H, Goodman A and Aldrich W, 1974. The thermomechanical properties of gutta-percha: III. Determination of phase transition temperatures for gutta-percha. Oral Surgery, Oral Medicine, Oral Pathology, 38(1), pp. 109-114.
Siqueira JF and Lopes HP, 1999. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. International endodontic journal, 32(5), pp. 361-369.
Siqueira JF, Favieri A, Gahyva SM, Moraes SR, Lima KC and Lopes HP, 2000. Antimicrobial activity and flow rate of newer and established root canal sealers. Journal of endodontics, 26(5), pp. 274-277.
Sirtes G, Waltimo T, Schaetzle M and Zehnder M, 2005. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy.Journal of endodontics, 31(9), pp. 669-671.
Sjögren U, Hagglund B, Sundqvist G and Wing K, 1990. Factors affecting the long-term results of endodontic treatment. Journal of endodontics, 16(10), pp. 498-504.
Sjögren U, Figdor D, Spångberg L and Sundqvista G, 1991. The antimicrobial effect of calcium hydroxide as a short‐term intracanal dressing. International endodontic journal, 24(3), pp. 119-125.
Tamse A, Unger U, Metzger Z and Rosenberga M, 1986. Gutta-percha solvents—a comparative study. Journal of endodontics, 12(8), pp. 337-339.
Ungor M, Onay EO and Orucoglu H, 2006. Push‐out bond strengths: the Epiphany–Resilon endodontic obturation system compared with different pairings of Epiphany, Resilon, AH Plus and gutta‐percha. International endodontic journal, 39(8), pp. 643-647.
Vertucci FJ, 2005. Root canal morphology and its relationship to endodontic procedures. Endodontic topics, 10(1), pp. 3-29.
Vertucci FJ, 1984. Root canal anatomy of the human permanent teeth. Oral surgery, oral medicine, oral pathology, 58(5), pp. 589-599.
In this article I hope to cover the development and evolution of a training programme covering dentistry and autism before moving onto the more practical aspects of dealing with autistic patients in a dental practice.
My own personal experience of delivering training in autism started more than a decade ago with some basic autism awareness raising and since then has evolved into a more specific dentistry and autism, delivered in a variety of settings and to various groups. I currently sit on NHS Highland’s Autism Strategy Group and participate in the Healthy Lives SubGroup. These groups are composed of a mixture of health, education and social care professionals and representatives of the autism community, either those with autism themselves or carers.
When considering dentistry and autism training there are various levels and facets to consider. Firstly, why is such specific training needed, to whom should such training be delivered, by whom, when and at what level?
Why is such specific training needed?
The current incident of autism diagnosis is increasing. There are various theories and explanations given for this rise, whether it be better diagnosis, earlier recognition or if there is an overall increase – it’s a multifaceted answer beyond the reach of this article. What is evident though is that the dental team is becoming more aware of the increasing numbers of autistic patients within all our practices. Autism is a spectrum condition; this means that the effects on an individual can vary from minimal to profound. There can be no affect on intellectual ability or a profound learning disability. There are various training packages available both nationally and locally (from the Open University and the National Autistic Society, to those run by local groups) but, in general, they deal with the condition itself only. If you are lucky, it will mention dentistry, but usually only briefly and then it will mention the difficulties involved.
What is required is training that highlights the needs and requirements of autistic patients and their carers within a dental setting. This training, while highlighting the main issues of autism, also needs to tackle those issues from a dental practice point of view.
To whom should training be delivered?
As part of NHS Highland’s Autism Strategy Group I recently surveyed local GPs on their experience of autism training. The results of this survey showed that, while most of those doctors had received some level of autism training, the reverse was true of their receptionists. This front-line element of dealing with autistic patients is vital. As such, we have ensured that when we are delivering training on any group of patients who may have additional needs that the training is delivered to the whole team, both clinical and administrator.
Those on the front desks are a vital element in reducing the stress and distress which can accompany dental visits by their communication skills and ability to adjust them accordingly. Within the dental setting it is imperative that all team members from front desk to surgery are included in any training.
While we train our dental teams on how to deal with an autistic patient, another aspect to be considered is providing training to local autism groups as to what they can expect and reasonably request in terms of visiting the dentist. Oral health advice leaflets can be distributed via drop-in centres and oral health messages incorporated into newsletters.
Who should deliver training?
During my own journey working with autism, I very quickly came to learn how political a community it can be. There are those who believe that all training on autism can only be successfully delivered by those with autism. Others are happy if there is consultation. We do, however, have to avoid the trap of producing a training programme and then sending it out for comments. This can leave people feeling uninvolved in the development of a programme, almost as if they are an afterthought.
There are numerous autistic societies throughout the country; some are national such as National Autistic Society while others will be more local. An example near myself is ARGH! (Autism Rights Group Highland!). A good relationship with both national and local groups can pay dividends in terms of disseminating oral health messages. We have training delivered by a dental team on its own, a dental team with autism diagnostician or a dental team with autism patient. Each has its own merits and advantages.
When should be training be delivered?
As with any training, this will depend upon need. Figures show the numbers of those with diagnosis is increasing. Whether that is due to better diagnosis, more diagnosticians or an absolute increase is still debated. As the Public Dental Service, we know that the number of referrals we are receiving for autistic patients is increasing, both adults and children. While some of these will be heading straight down a GA pathway due to their level of co-operation and ability to communicate, many others are for general dentistry.
As such, each practice should be aware of their own needs for training and arrange as frequently as they see fit. Ideally, new staff should be given training as soon as they start. Refresher training will depend upon your need – paradoxically it may be that if you do not deal with autistic patients often, you will require refresher training more frequently as, with so many things learned on courses, if you don’t use it you lose it.
At what level should training be delivered?
There are two aspects to training in dentistry and autism, there is the general awareness-raising of autism and its associated traits and comorbidities, and then there are the elements specific to dentistry. All healthcare professionals should be aware of autism and how it may affect patients, but surprisingly few have ever been given any formal training in this. Attending any meeting on autism and health, it soon becomes apparent that the bulk of most agendas is actually about diagnosis services rather than interacting with all the other elements of healthcare.
With regard to the elements specific to dentistry, the exact level will depend upon your patient base. A community clinic, which is seeing more complex cases will require more in depth training than a GDP practice would. Such additional training may include work on consent, working under GA, violence and aggression training, including the use of clinical holding.
How should training be delivered?
Training is available from a variety of sources, and there are numerous online packages at various prices. These are mostly very general in nature and, if you are lucky, may mention dentistry in passing. The Scottish Government has recently been funding professionals through an Open University Understanding Autism module, but within it there is no mention of dentistry at all. However, for an overview of autism it is very useful. The best for dentistry, therefore, is a specific training session, which can be as short as an hour or as long as an entire day. They are, however, few and far between. The ideal is an in-practice session attended by all staff members, not just the clinical team but also the receptionists.
Accumulation of marginal gains
I shall now explore various hints, tips and strategies that can help when dealing with patients on the Autistic Spectrum and their carers. I shall highlight a number of areas where small changes can together combine to bring about a more successful outcome. The phrase “accumulation of marginal gains” came into popular culture during the 2012 Olympics as it was the mantra of the Team GB Cycling team. The philosophy is that there is unlikely to be any one major change which gives you a revolutionary step ahead, but by bettering performance in a multitude of minor areas it will give an overall improvement.
As such, we will explore the options where we can make such small changes in the hope that they can combine to give us, as a dental team, a better chance of managing and treating autistic patients.
Medical questionnaires
On the basis that forewarned is forearmed, we include a specific autism question on our medical questionnaires. The question reads: “Are there any other details or conditions which it may be helpful for your dentist to know about but are not mentioned e.g. autism or Aspergers syndrome, physical or learning disabilities.” This gives patient and carers a chance to prewarn us of a diagnosis or even prediagnosis.
We operate a helpline system for patients which asks broader questions. They include: “Does the patient have any medical condition which you feel has a negative effect on their dental health” and “Do they have any condition which affects their ability to care for themselves or seek care for themselves”. These are two very crude questions but are specifically designed to capture autism so that those patients can be directed to an appropriate clinic.
Once we are aware of the fact that a patient has autism, we can then take measures to gain additional information to aid in our interactions with them.
Pre-visit questionnaires
Once it is known that a potential autistic patient will be attending for the first time, we send them a pre-visit questionnaire. We have two different versions, an adult and a child. They basically ask the same questions but with slightly different wording. The questions ask about various aspects that would make visits easier: sensitivities, best time of day for an appointment, any expected difficulties, with communication and anything else that might help us.
The questionnaire is a single side of A4 which can be easily answered in as little or as much detail as a patient or carer wishes. However, the information contained can be invaluable in ensuring that a visit goes smoothly.
Pre-visit packs
As well as a questionnaire, we offer to send out a pre-visit pack to those attending for the first time. These can be especially useful to children. The contents of the pack can vary but essentially depend upon the ingenuity of the person assembling it. Our usual pack contains a pair of gloves, a mask, a disposable mirror, a cotton wool roll and a sticker. This has the value of being compact enough to fit within a standard envelope and thus keep postage costs down.
Appointment timing and length of appointments
Unsurprisingly, the timing of an appointment can have a significant impact on the success of an appointment. This can be due to the actual timing and how it fits into a patient’s normal daily routine, but also how that person copes with change at different times of the day. Some autistic patients will be better first thing in the morning, while others may be better at the end of the day. This information will only come from direct contact and questioning. As such, blind sending out of appointments may not result in a success.
The other issue around timings of appointments is the impact a full or semi-full waiting room may have upon an autistic patient, as well as the physical wait itself. Ideally, there should be no waiting as this can cause stress and distress to many on the spectrum. It may be better to schedule such patients right at the start or right at the end of a session when there is unlikely to be many in the waiting room.
If the end of the session is chosen, then care should be taken to ensure that appointments are running to time. We encourage those for whom waiting is an issue to contact us shortly before their appointment to ensure we running on schedule. We also allow patients to contact us to tell us they have arrived but are waiting in the car park until we are ready, thus eliminating any need for sitting in a waiting room at all.
We usually allow a slightly longer appointment time for autistic patients, not necessarily for the treatment itself but to allow time for the patient to become comfortable in unfamiliar surroundings and because often communication can take longer.
Minimal arousal environment
When treating autistic patients we try to have a minimal arousal environment. One of the main issues with autism is a hyper or hyposensitivity, and this can be to external or internal sensations. This means there can be unusual reactions to lights, noises, tastes or movements. Therefore, we can turn off overhead lights, especially if they are fluorescent tube lights as these can cause hypersensitivity due to flicker. We also turn off the radio and we can turn off the ringer on surgery phones and place a notice on the door to prevent interruptions.
With regard to taste, plain water may be appreciated as a mouth rinse rather than mint, orange or thymol. Movement and balance is an often overlooked aspect of autism but we can help by having the chair in a reclined position thus preventing the need to have the patient moving in the chair.
Social stories
Social stories are a form of pictorial storyline often used in other fields such as education. I was, in fact, introduced to them by my wife, who is a teacher. They compromise of a series of pictures accompanied by short text. They can be photographs or cartoons and can be used to represent a variety of different topics from a general exam visit to a more specific treatment such as fissure sealants.
A simple example might be: “This is the dental clinic where I will go to have my teeth counted” with a picture of the building. “This is the room I will sit and wait until it is time for my teeth to be counted,” with a picture of the waiting room. “This is the chair I will sit in to have my teeth counted,” with a picture of the dental chair. And: “The dentist will wear gloves and use a mirror to count my teeth,” with a picture of gloves and a mirror (this ties into the pre-visit pack). “This is the dentist who count my teeth,” alongside a picture of the dentist and, finally: “After my teeth are counted I will get a sticker,” with pictures
of stickers.
The principle is very simple, positive language with repetition reinforced by pictures. With modern cameras, they are extremely easy to produce by any member of the team and can be used for many different patients not just autistic ones.
Special interests
As a query on the pre-visit questionnaire, we ask about special interests. Often, autistic patients will have a favourite topic which they like to talk about or are interested in. If we know what this is then we can utilise it to our advantage with suitably themed rewards.
We had one such patient who liked lights, so we let them play with our dental light after an exam if they let us examine their teeth. Another required the procurement of a number of large elastic bands, but more often it is just suitable stickers or colouring sheets of favourite TV characters.
Distracters and comforters
Often, autistic patients will already be armed with their own objects for distracting or comforting them. This may be as simple as a set of headphones, either noise cancelling or playing music to more complex such as iPads or portable devices. It may also include favourite items such as toys, pieces of clothing, rope etc.
We should allow any patient who wishes to continue using any such objects – unless they directly interfere with treatment. Some autistic patients derive a degree of comfort during stressful periods from what is known as “stimming” – short for self-stimulation. This is basically any form of hand movement and may or may not involve an object of some sort. Therefore, it may be useful to have squeezy stress balls available that can be offered to those who might benefit from them.
Communication
We can help autistic patients by adjusting the way that we communicate and there are a number of simple strategies that can be of assistance.
Say the patient’s name first to get their attention. So it is: “John, please open your mouth” rather than: “Please open your mouth John.”
We may consider reducing our language, using only key words, such as: “Music off” rather than “Please turn off the music now.”
We want to say things in the order that they will occur, so it’s: “Glasses on, then sit on the chair, then teeth counting.” The word “then” is a good link word that is easily understood.
Giving clear choices will help understanding, so a question such as “Which sticker would you like?” becomes far easier when it is reduced to “Barbie or Scooby Doo?”
We should always try to give positive instructions rather than telling patients what not to do, so instead of: “Don’t run around the chair” it is preferable to say: “It’s time to sit in the chair.”
A concept that occurs frequently with an autistic patient is the importance of being told when something is over. For this, it can be useful to use the word “finished”.
If we use it consistently, this will help the patient understand the concept of time and help to keep them calm.
Summary
The above is a very brief summary of a large number of tactics that can be deployed to use when treating autistic patients. However, they are not always successful and, as such, we may have to refer the patient onwards for treatment under GA. This brings its own challenges but ultimately is often the only option for some patients.
But, by taking our time and adjusting how we prepare for our patients, how we change our environment and how we communicate we can have success and make visits
to the dentist a lot less stressful that it might otherwise have been.
I wish you all the success in future dealings with this group of patients.
There is unlikely to be one major change that gives you a revolutionary step ahead
Useful links
- NES Learning Resource http://asd.nes.scot.nhs.uk/
- Knowledge Network http://www.knowledge.scot.nhs.uk/home/ learning-and-cpd/learning-spaces/autism-spectrum-disorder.aspx
- National Autistic Society advice for dentists http://www.autism.org.uk/professionals/ health-workers/dentists-info.aspx
- National Autistic Society – Going to the dentist https://www.autism.org.uk/advice-and-guidance/ topics/physical-health/going-to-the-dentist/dentists
- British Society for Pediatric Dentistry Advice (PDF)
About the author
Malcolm Hamilton works as a senior dental officer in NHS Highland’s Public Dental Service. He has worked there since 2009, prior to that he was a senior dental officer in NHS Orkney. He qualified from Dundee in 1989 and moved to Orkney after serving in HM Forces. He currently works in Sutherland and Easter Ross providing a range of dentistry to his patients there. He has a special interest in autism and in domiciliary dentistry.
Malcolm can be contacted by email: malcolm.hamilton@nhs.net
This article seeks to look at chronic oral facial pain and the stress experienced by the dental team. This work will present evidence that in many cases both of these states are manifestations of the dis-ease in a divided mind. In chronic oral facial pain, we will examine the conditions of TMD, burning mouth and atypical facial pain from the mind body perspective. The work of John Sarno will be explored as he seeks to offer an explanation of how the brain diverts unconscious rage into physical symptoms without consulting the conscious rational mind.
The research by Goldthorpe et al (2016) offers an interesting complex intervention model based on CBT (cognitive behavioural therapy) which embodies many of the ideas proposed by Sarno. Linking the dis-ease of chronic oral facial pain and the pervading stress in our profession will be the Adverse Childhood Experiences (ACE) studies. Dr Robert Block, the former president of the American Academy of Pediatrics, purports that the results and implications of the ACE study, are the single greatest unaddressed public health threat facing America today. What is true in America is also true in the UK, as the ACE studies in Wales and England have demonstrated. The studies, which look at how adverse childhood experiences impact on all aspects of adult health, both physical and mental, is backed up by robust scientific evidence.
In light of this evidence, Gabor Mate’s (2003) seven A’s of healing hold great relevance for ‘health as a team sport’ approach. The pains, ills and addictions of our modern society are a sign of the great disconnects that both health providers and patients are experiencing in their lives. When a boy child with Attention Deficit Hyperactivity Disorder would be president of a major world power it is time to sit up and pay attention.





The cost of chronic pain
Let me begin with the potential cost to both the patient and the health service of ignored somatised pain. Late one Friday night, at an early stage in my career I was asked to attend an accident and emergency department at half past midnight. I had been awakened from my slumber by a young casualty officer who requested that I remove an anterior four-unit bridge from a very distressed lady. On entering the department, I was confronted by a 62-year-old anguished lady who had attempted suicide. Her mutterings were partly comprehensible and I learned that she’d had intractable pain in her anterior front teeth since the four-unit bridge had been fitted two years previously.
In a faltering voice influenced by her torment she revealed that no one would believe her when she had explained the intensity of the pain and how it was impacting on all areas of her life. In the last few months she had become fixated on her bridge and believed that if this was removed then the pain would disappear. She kept uttering “no one would believe me, no one would believe me”. I gently said that I believed her and if it was her wish I would remove her bridge.
On clinical examination, it was an aesthetically and technically good anterior fixed appliance and my heart sank at the prospect of removing it. With her written consent, the bridge was finally set free from her oral cavity and she was released from the dental chair into the waiting care of the psychiatry department.
That whole episode has remained with me over the years and has informed my disposition to patients who present with pain but with no obvious organic cause. It can be difficult to resist intervening when a patient presents with intractable symptoms but in the absence of pathology, resistance is the better part of valour. In dental practice it is not uncommon to encounter patients who have chronic oral-facial pain of a non-odontogenic origin. This pain may take the form of tempero-mandibular dysfunction, burning mouth syndrome (oral dysathesia) or atypical facial pain.
After a careful and thorough history into the nature and distribution of the pain the patient is often referred to an oral surgery or oral medicine department. Within these departments, after thorough investigation which may include expensive tests, the patient is often given treatment in line with the biomedical model. For many patients with chronic pain, research now shows psychological factors play a role in the development, exacerbation and maintenance of their symptoms. Even though this is recognised by many clinicians, there are few psychosocial models in place to offer long-term resolution for this cohort of patients.
The pain is real
Dr John Sarno, an American physician, wrote a best-selling book on healing back pain, where he describes tension myositis syndrome (TMS). He proposes that the pain from this syndrome is caused by unconscious repressed rage and the diversion of this rage into somatic symptoms. The pain, he states with great emphasis, is real, as demonstrated by eliciting tenderness on palpating the affected muscle groups. He goes onto describe how the pain experienced by the patient is mediated by the autonomic-peptide system often by way of a decrease in oxygen to the affected muscle group.
The somatisation of psychological distress has been described since the time of Charcot, Breur and Freud and was written extensively about in Studies on Hysteria. They describe the split between the conscious rational mind and the more childish primitive unconscious mind. The unconscious mind is often described in terms of the shadow and holds all the emotions that we fear to look at consciously. Take the parapraxis, also known as the Freudian slip, where the unconscious repressed wishes reveal themselves to the light of day. They can often be funny anecdotes but they do reveal part of what is repressed. For example, the slip of tongue that states “A Sale of Two Titties” instead of “A Tale of Two Cities” (see Fig 1).
The masseter muscle is the strongest muscle in the body, helping to exert upwards of 200 pounds of force when the molar teeth are clenched. It is also the holder of a great deal of tension with the unconscious nocturnal grinding of teeth and the diurnal clenching of teeth. The question is, what is the purpose of this grinding and clenching which can lead to micro fractures of the teeth and hypertrophy of the masseter muscles? The pain from this muscle tension can cause great distress to patients impacting on all areas of their life.
Patients often report that when their life is more stressful this pain increases in intensity. The Dr Sarno methodology counsels patients that this pain is real and, indeed it is, but the source of the pain is psychological as opposed to physical. If a patient can accept this fact and acknowledge that it is due to repressed emotions, then the conditions for the reduction in pain and even cure are set in place. Secondly, and just as importantly, it is essential that the patient familiarises themselves with the sources of their repressed emotions. Sources can include childhood events, high expectations of themselves, perfectionism, inner criticism, sensitivities, people pleasing, worrying, the need to be good, responsibilities – the list is endless.
The patient can be given instructions on jaw massage and exercises to ease the muscle tension but the definitive treatment is recognising the emotions that the patient is afraid to bring into the light of day. Techniques such as mindfulness are good to identify emotions but it is important that the patient becomes aware of the real source of these emotions. This would not only help to ease their TMD but their greater awareness could offer the opportunity to a far richer life.
Complex intervention
The research by Goldthorpe et al (2016) offers an interesting complex intervention model based on CBT which may prove to be of great value to TMD and patients with chronic oral facial pain.
There are to date no clinical guidelines to offer definitive care pathways to patients suffering from chronic oral facial pain. This is perhaps because the true extent of the mind-body connection is not fully acknowledged. Future research in this domain is necessary in order to relieve the distress of this increasing number of patients.
The complex intervention model need not be complex at all, as the primary purpose of it is to gently educate the patient as to a plausible explanation for their symptoms. This education is based primarily on believing fully the extent of the symptoms and the impact they are having on the life of the individual. From this starting point of unconditional acceptance, the long-term management of the condition can be forged in a collaborative environment. There is much research work to be done in the mind body spirit connection in order to reach health and balance for this group of patients.
ACE Study
Epidemiological research connecting early childhood experience to the later development of physical and psychological diseases may offer answers to chronic oral facial pain, and the stress many of the dental team will experience in their lives. The ACE study was carried out in 1995 and 1997 in California by Kaiser Permanente, a private health company, and the Centers for Disease Control and Prevention. The 17,337 participants have been followed up regularly ever since and monitored for their experience of health and well being. The average age of participants was 57, with 74.8 per cent being caucasians and
75.2 per cent had a college education. All had jobs and good health care, demographics which would closely parallel the dental team in this country. The study asked 10 questions enquiring whether a person had experience of any of the following conditions during childhood:
- physical abuse
- sexual abuse
- emotional abuse
- physical neglect
- emotional neglect
- mother treated violently
- household substance abuse
- household mental illness
- parental separation or divorce
- incarcerated household member.
One adverse childhood experience was reported by about two-thirds of the participants. The number of ACEs was highly correlated with adulthood behaviours such as smoking, alcohol, drug-taking, obesity, promiscuity and ill health including depression, heart disease, cancer and chronic lung disease. The more ACEs the more likely a shortened life span in adulthood (Fig 2). The neurobiology of stress in childhood offers an explanation of how these negative consequences may occur. Under stressful conditions, neural networks are altered along with the biochemistry of the neuroendocrine system.
The scientific evidence to support how early deprivation alters neuro pathways is now firmly established and unless these deficiencies are addressed the damage acts like chaos theory later on in life.
Forging a new path
The good news for our patients and for our own personal wellbeing is neuroplasticity. The ability of the brain to form new neuro pathways can now be demonstrated by neuroimaging. The work of Lindon (2006) has shown how psychotherapy can alter brain pathways in conditions such as depression and post traumatic stress disorder. I am not advocating mass uptake of psychotherapy, but I am suggesting that the more conscious we become and the more aware of our lives we become then the richer our experience of life will be.
Socrates stated that the unexamined life was not worth living and, in fact, he was willing to die for that. But what is meant by that in our personal lives and indeed our professional lives? The GDC in its Standards for the Dental Team and their document Developing the Dental Team both mention reflective practice. Yet, do we know the physiological, cognitive and psychosocial skills necessary to truly reflect?
We live in very fast and furious times, connected day and night to cyberspace and external stimulation. Is it any wonder that many of us feel disconnected from what really matters in life? The good life or happy life has been the focus of philosophy and psychology from the beginning of time. Much wisdom has come to us from these sources but we have to slow down to listen to the wisdom of our own hearts. There is much to be said for finding a quiet time in our busy schedules to develop the art of mindfulness. Mindfulness has been born again from its ancient origins in the East. It can be thought of as a simple form of meditation which if practiced regularly has been shown to decrease stress and lessen the symptoms of depression.
At it simplest form, the practitioners of this art seat themselves in a comfortable chair with plenty of support for the back. If doing this in a busy day, it can be good to set the alarm on your mobile phone so that your are not distracted worrying about time. It is good to close your eyes and listen to the sound of your breathing and become aware of any sensations in your body. While doing this, you will notice that thoughts may arise; notice them with compassion, but do not become attached to them, as thoughts are passing. If you are tempted to dwell on the thoughts, return to your breathing, noticing your inhalations and expirations.
In a busy surgery the practice of mindfulness together on a daily basis can enable each team member to see the world with a greater clarity. Do not worry about becoming some Zen-like monk, the practice of mindfulness enables you to cultivate a deep compassionate awareness which allows you to assess your values and goals. There are many courses available to help you with mindfulness, I believe NES even facilitates one.
Jon Kabat Zinn, emeritus professor of medicine and creator of the Stress Reduction Clinic and the Center for Mindfulness in Medicine has been credited with bringing this ancient art into modern medicine. He is quoted as saying that “You can’t stop the waves but you can learn to surf”. YouTube has many helpful videos to get you started on this life changing path.
Face your reality
In the quest to reduce stress it is good to remember the famous maxim: “If you always do what you’ve always done you’ll always get what you’ve always got.” There is great wisdom in this quote. If we are not prepared to look at ourselves, then things will continue as they are. It is not life that makes us stressed, it is the way that we respond to life. How we respond to life is chiefly influenced (as borne out in the ACE studies) by our early childhood conditioning.
Gabor Mate who wrote the book When the Body says NO, exploring the stress-disease connection, advocates what he terms the seven As of healing. These are: Acceptance, Awareness, Anger, Autonomy, Attachment, Assertion, and Affirmation. True reflection requires that we are able to accept ourselves as we truly are, not as we would like to be but as we are in this moment in time. Change is only possible after acceptance and acceptance is only possible if we are able to have compassionate curiosity about ourselves and other.
When I talk about acceptance I do not mean passive acquiescence but the ability to look at myself and my situation as it truly is. Facing and acknowledging our reality brings much greater awareness and means we can be fully present and responsible. We accept responsibility for ourselves and the experience we are having at this time whether it is a good or a bad experience.
Accepting yourself in the present moment means you learn to let go of the thoughts that say “you are bad or good, hopeful or hopeless”. You just are. You show up as you are letting go of all the things you fear to lose.
The second wish to a stress-free life is to become more aware. Awareness is the ability to recognise the physiological signals within your own body and the ability to interpret the emotional truth in the other. How many times have you had a gut feeling that you should have done one thing and only realised the cost of not heeding that after the event.
There is not enough space to go into the other As but each of them if engaged with sincerely offers a way of gaining greater understanding of our inner world. Carl Jung, the great Swiss psychiatrist, is quoted as saying “Who looks outside, dreams; who looks inside, awakes”. It is only in the depths of our inner world that we will discover the wealth of our being. Mindfulness is a great starting point, but in order to undergo long-term change we must be prepared to explore the uncharted waters of our inner life.
Conclusions
Taking a more pragmatic stance, there are things we can do to reduce stress but they must be done on a consistent basis. It goes without saying a healthy diet, regular exercise, and sleep are essential to reduce stress. In fact one of the main signs of stress is that we give up doing the things that are good for us and the things that give us pleasure. In times when life is difficult it is often the wisdom of poets who help us to see new light.
I leave you with the words of Max Ehrmann and invite you to take a seat and slowly listen:
Desiderata: Words for Life
Go placidly amid the noise and haste,
and remember what peace there may be in silence.
As far as possible without surrender
be on good terms with all persons.
Speak your truth quietly and clearly;
and listen to others,
even the dull and the ignorant;
they too have their story.
Avoid loud and aggressive persons,
they are vexations to the spirit.
If you compare yourself with others,
you may become vain and bitter;
for always there will be greater and lesser persons than yourself.
Enjoy your achievements as well as your plans.
Keep interested in your own career, however humble;
it is a real possession in the changing fortunes of time.
Exercise caution in your business affairs;
for the world is full of trickery.
But let this not blind you to what virtue there is;
many persons strive for high ideals;
and everywhere life is full of heroism.
Be yourself.
Especially, do not feign affection.
Neither be cynical about love;
for in the face of all aridity and disenchantment
it is as perennial as the grass.
Take kindly the counsel of the years,
gracefully surrendering the things of youth.
Nurture strength of spirit to shield you in
sudden misfortune.
But do not distress yourself with dark imaginings.
Many fears are born of fatigue and loneliness.
Beyond a wholesome discipline,
be gentle with yourself.
You are a child of the universe,
no less than the trees and the stars;
you have a right to be here.
And whether or not it is clear to you,
no doubt the universe is unfolding as it should.
Therefore be at peace with God,
whatever you conceive Him to be,
and whatever your labors and aspirations,
in the noisy confusion of life keep peace with your soul.
With all its sham, drudgery, and broken dreams,
it is still a beautiful world.
Be cheerful.
Strive to be happy.
ABOUT THE AUTHOR
Mary graduated from Glasgow University in dentistry in 1980 and from the Open University in psychology in 2001. She obtained a postgraduate diploma in counselling and psychotherapy from Stirling University in 2013 and a postgraduate diploma in clinical education in 2017. She has enjoyed a plethora of experiences in dentistry, both in the UK and abroad. Mary is now in full-time psychotherapy practice. If you would like to contact her with regard to anything in this article, please email marybdownie1@gmail.com or call 07970 909 373.
References
- Adverse Childhood Experiences (ACE) Study (Web Archive)
- CDC – Ace study
- Block, R – Who needs to pay attention to the ACE study (Georgetownta.wordpress.com) – accessed on 23/05/2013
- Breuer J, Freud S. Studies on Hysteria, 1895.
- Goldthorpe. J, Peters. S, Lovell. K, McGowan. L, and Aggarwal. V. ‘I just wanted someone to tell me it wasn’t all in my mind and do something for me’: Qualitative exploration of acceptability of a CBT based intervention to manage chronic orofacial pain. British Dental Journal, Volume 220, No. 9 (May 13, 2016)
- Linden, D.J. 2006. How psychotherapy changes the brain – the contribution of functional neuroimaging. Molecular Psychiatry, 11, 528-538
- Mate, G.‘When the Body says NO, exploring the stress-disease connection’. Alfred A. Knopf Canada (2003)
- Sarno, J. The Divided Mind: The epidemic of mind body disorders. Duckworth Overlook, London (2008)
Obtaining valid consent
The risk of anti-fungal resistance in dentistry: lessons from a Japanese fungus
Globally since 2009, Candida auris, a fungal species closely related to Candida albicans, has been responsible for a number of… Read more
The anxious patient: Empathy, planning and a team-approach
[ Words: Ilyaas Rehman, BDS (Glas) ] Introduction Introduction This case report documents the treatment provided for a very anxious… Read more
Meaningful milestone
Consultation represents the next step on the journey towards achieving a more effective system of CPD Some dental professionals feel… Read more
Top tips for practicing evidence-based dentistry: Part 3
Introduction Implementing the best available evidence and enabling positive sustainable change in practice is an enviable goal for anyone providing… Read more
The management of a paediatric dental avulsion in general dental practice
Abstract A paediatric dental avulsion is not a clinical scenario that you will be faced with on a daily basis… Read more
Intraoral scanners In dentistry – an update on digital technology
With the introduction of the first intraoral scanner, CEREC (Chairside Economical Restoration of Esthetic Ceramics) by Dentsply Sirona in 1985,… Read more
CBCT and clinical decision-making
Arvind Sharma, BDS(Dund), MSc(Endo), MJDFRCS(Eng), MFDSRCPS(Glas) Arvind Sharma presents the second and final part of a structured critical review to… Read more
Problems of a phobic patient
Day One Ms B attends as a new patient at a local dental surgery complaining of sensitivity in a number… Read more
The power of regeneration
Due to its powerful regenerative properties, platelet rich growth factor Endoret (PRGF) is being increasingly used in many fields of… Read more
Suitability of patients for conscious sedation
Patients suitable to undergo conscious sedation (CS) include those with moderate-severe anxiety, a swallow/gag reflex or a mild learning/physical disability… Read more
Top tips for practicing evidence-based dentistry
In the first article, we explored how to establish a research question using the PICO (Population, Intervention, Comparator and Outcome) method… Read more
Quick, aesthetic restoration
A new patient in her early 30s attended for a check-up. A routine radiograph revealed caries under the amalgam filling… Read more